Active surveillance offers a safe, patient-centred option for men with localised, low, or favourable intermediate-risk prostate cancer
Prostate cancer represents 15 per cent of all cancers and is the second most common manifestation of the disease in men worldwide. The average age of diagnosis is 66 years.
Most prostate cancers are found when the disease is in only the prostate and nearby organs and the five-year survival rate for most people with local or regional disease is nearly 100 per cent.1
Prostate cancer in Ireland
In Ireland, prostate cancer is the most commonly diagnosed cancer in men, with around 4,000 new cases each year. Over three-quarters of men are diagnosed with localised, low- or intermediate-risk disease, often without symptoms at the time of detection. This translates to a one-in-nine risk of developing prostate cancer before the age of 75.
Ireland ranks seventh globally and fourth in Europe in age-adjusted prostate cancer incidence rates, with 99.8 cases per 100,000 population annually.2 From 2019 to 2021, prostate cancer accounted for an average of 623 deaths each year nationally. The Irish Government has prioritised cancer care, investing over €456 million since 2017, and subsequently improving diagnosis, treatment infrastructure, and staff capacity.
The National Cancer Strategy (2017–2026) and the National Cancer Control Programme (NCCP) guidelines promote active surveillance (AS) for suitable prostate cancer patients, building on international best practice and evidence.
What is AS?
AS is a structured protocol of ongoing monitoring for men diagnosed with low- to intermediate-risk prostate cancer aimed at avoiding or delaying curative treatment (eg, surgery, radiotherapy) unless disease progression is detected.
Its goal is to preserve quality-of-life by avoiding treatment-related side-effects whilst conserving the window of curability, initiating active treatment only when clinically necessary. AS differs from watchful waiting in that AS seeks not to lose the patient’s curative window (Table 1).3
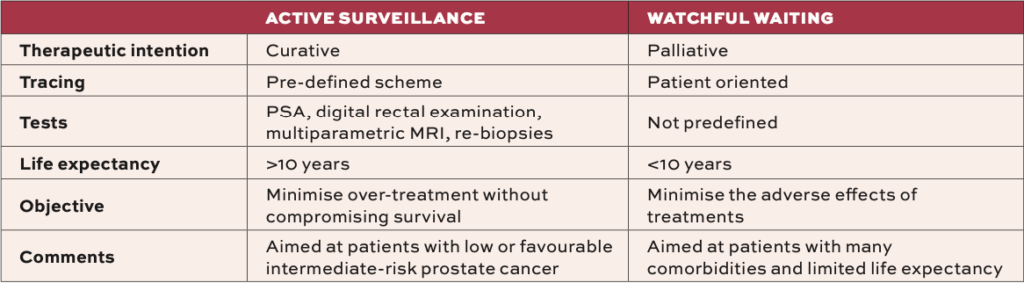
Watchful waiting is more palliative, for older or unfit men, focusing on symptom management. Much evidence for AS comes from the Canary Prostate Active Surveillance Study (PASS), which is a multi-centre, prospective cohort study that initiated in 2008 in response to mounting evidence of over-treatment in many men with prostate cancer. A sample population of 2,155 men with favourable-risk prostate cancer and no prior treatment were enrolled at 10 North American centres.
Data analysis revealed that 10 years after diagnosis, 49 per cent of the men remained free of progression or treatment, less than 2 per cent developed metastatic disease, and less than 1 per cent died of their disease. Later progression and treatment during surveillance were associated with worse outcomes, and the results demonstrated that AS was an effective management strategy for patients diagnosed with favourable-risk prostate cancer.4
Guideline updates
The NCCP issued an update on the HSE National Clinical Guideline ‘AS for patients with prostate cancer’ in April.
The purpose of the national guideline is to provide evidence-based recommendations on the AS of patients with prostate cancer through the integration of the best research evidence with clinical expertise, patient values, and experiences. As well as addressing areas of care with new and emerging evidence, the guideline also aims to reduce variation in practice and improve patient experience and service delivery. The following are the key recommendations:
- All patients must have undergone pre-biopsy magnetic resonance imaging (MRI), followed by systematic and targeted biopsies prior to consideration for AS.
- Eligibility criteria:
• Any Gleason 3+3 (Grade Group 1), prostate-specific antigen (PSA) <20μg/L, ≤cT2.
• Any Gleason 3+4 (Grade Group 2) in the absence of intraductal or cribriform pattern, PSA <20μg/L, ≤cT2. For this group further considerations must be assessed including: If the patient has had a targeted biopsy; percentage of positive core; percentage of pattern 4; pattern 4 subtypes; and PSA density.
The Guideline Development Group agree that there is a paucity of high-quality evidence on risk stratification for patients with prostate cancer being considered for AS. Therefore, their consensus is to recommend the most current three-tier STRATCANS stratification and follow-up strategy.5
This strategy evolved from a single centre prospective study that reported on the implementation of the three-tier STRATified CANcer Surveillance (STRATCANS) follow-up strategy.6
This strategy has used UK National Institute of Health and Care Excellence (NICE) recommended Cambridge Prognostic Group (CPG) 1 or 2, PSA density (PSAd), and MRI PI-RADS (prostate imaging-reporting and data system) score at entry, to identify patients at three different risks of disease progression. This tiered system has tailored the intensity of follow-up (Table 2).6
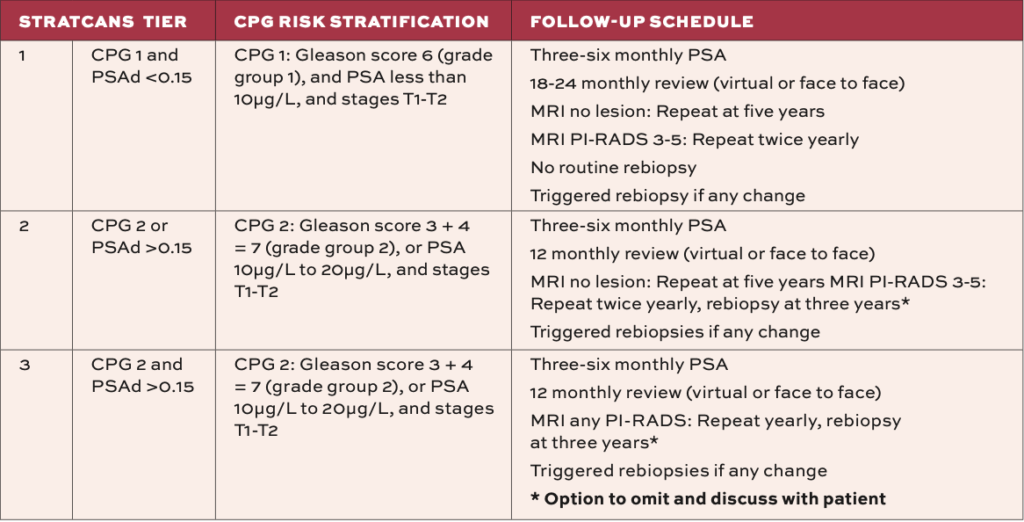
Trigger points for proceeding to treatment include rising PSA, MRI findings suspicious for progression (eg, new PI-RADS 4 lesion), biopsy upgrade (eg, Gleason 4 pattern or higher volume), and clinical findings on DRE. The NICE guideline also outlines that an important trigger for conversion to radical treatment may be patient preference.6
NICE recommends that if a person wishes to move from AS to radical treatment at any stage in their care, a shared decision-making approach should be used, taking into account the person’s preferences, comorbidities, and life expectancy.7
AS: The benefits and risks
As with any management strategy, there are benefits and risks associated with choosing AS. The patient must be fully informed and understand this from the outset. The NCCP recommends that patients entering AS should have access to a clinical nurse specialist or advanced nurse practitioner to discuss the benefits and harms and support shared decision-making of long-term AS and deferred treatment.5
All patients offered AS should receive written information detailing what is involved in the AS protocol and details of the triggers of possible cancer progression. All patients should be informed about cancer support groups and also be offered an education session virtually, face-to-face or with written material through an advanced nurse practitioner or clinical nurse specialist.5
The benefits of AS include the avoidance of treatment side-effects, especially erectile dysfunction, urinary incontinence, and bowel issues; maintenance of quality-of-life with minimal lifestyle disruption; research proven low rates of metastasis/death in properly monitored cases; and cost-effective care by reducing overtreatment and resource use.
Risks include the psychological burden of living with untreated cancer, which can cause anxiety. Patients generally believe that cancer is inherently life-threatening, and so the perception that they would not be receiving curative treatment for their cancer causes significant anxiety for some.8 Invasive follow-up tests including biopsies with infection/bleeding risks and the realistic variation in access are also negative aspects.
AS in Ireland: The data so far
The Irish Prostate Cancer Outcomes Research (IPCOR) registry was established in 2015 to provide evidence-based data on men with prostate cancer in Ireland and better inform care of the disease. Data to date demonstrates that men in Ireland have access to multiparametric MRI imaging, with almost 80 per cent of all men ultimately receiving one. However, only a minority had their MRI before their biopsy, and in most cases, the MRI was not used to direct their biopsy. Those who attended private hospitals with insurance were three times more likely to have a pre-biopsy MRI, allowing for targeted biopsies. This demonstrates unequal and untimely access to MRI imaging in Ireland.9
Conclusion
To summarise, choosing AS as a management approach may be best suited for men who have confirmed low or favourable intermediate-risk prostate cancer via biopsy and MRI and wish to preserve quality-of-life and avoid treatment side-effects. Patients must commit to regular follow-up appointments including PSA, MRI, and biopsies, and understand and accept the small risk of progression and possible anxiety involved. Diagnosis must include a high-quality baseline assessment (MRI, Gleason score) and patients should receive a clear follow-up plan. It is essential that there is easy access to support services, including a clinical nurse specialist or advanced nurse practitioner. Finally, patients must be confident that if progression occurs, curative treatment options remain viable and accessible.
AS in Ireland for prostate cancer reflects a thoughtful, evidence-based approach that balances early cancer control with preservation of life quality and minimisation of over-treatment. Supported by national guidelines, it offers a safe, patient-centred option for men with localised, low, or favourable intermediate-risk disease. With continued investment and research, AS in Ireland is well‑placed to remain a leading model of prostate cancer care.
References
1. Touijer K. Prostate cancer, basics on diagnosis. Urology Cheat Sheets [Internet]. Memorial Sloan Kettering Cancer Centre, New York. 2021. Available at: https://urologycheatsheets.org/sheet/prostate-cancer-basic-on-diagnosis-by-karim-touijer/
2. National Cancer Registry Ireland. Cancer in Ireland 1994-2021: Annual statistical report of the national cancer registry. NCRI; 2023. Available at: www.ncri.ie/en/reports-publications/reports/cancer-in-ireland-1994-2021-annual-statistical-report-of-the-national
3. Gonzalez D. Active surveillance in prostate cancer [Internet]. 2021. Available at: https://urologycheatsheets.org/wp-content/uploads/2021/01/Vigilancia-activa-UCS14302-v2-ENG14970.pdf
4. Newcomb LF, Schenk JM, Zheng Y, et al. Long-term outcomes in patients using protocol-directed active surveillance for prostate cancer. JAMA. 2024;331(24):2084-2093
5. HSE/NCCP. National Clinical Guideline for the Diagnosis, Staging, and Treatment of Prostate Cancer. 2025. Available at: www2.healthservice.hse.ie/organisation/national-pppgs/hse-national-clinical-guideline-active-surveillance-for-patients-with-prostate-cancer/
6. Thankapannair V, Keates A, Barrett T, et al. Prospective implementation and early outcomes of a risk-stratified prostate cancer active surveillance follow-up protocol. Eur Urol Open Sci. 2023;49:15-22
7. National Institute for Health and Care Excellence. Prostate cancer: Diagnosis and management [Internet]. London: NICE; 2019 (updated 2021). (Clinical guideline [CG131]). Available at: www.nice.org.uk/guidance/NG131
8. van den Bergh RC, Essink-Bot ML, Roobol MJ, et al. Anxiety and distress during active surveillance for early prostate cancer. Cancer. 2009;115(17):3868-3878
9. Gordon N, Dooley C, Murphy Á, et al. Irish Prostate Cancer Outcomes Research (IPCOR) registry: Cohort profile. BMJ Open. 2024;14(12):e090207










Leave a Reply
You must be logged in to post a comment.