Reference: June 2025 | Issue 6 | Vol 11 | Page 10
The European Alliance of Associations for Rheumatology (EULAR) recently published updated recommendations on the treatment of systemic sclerosis (SSc).
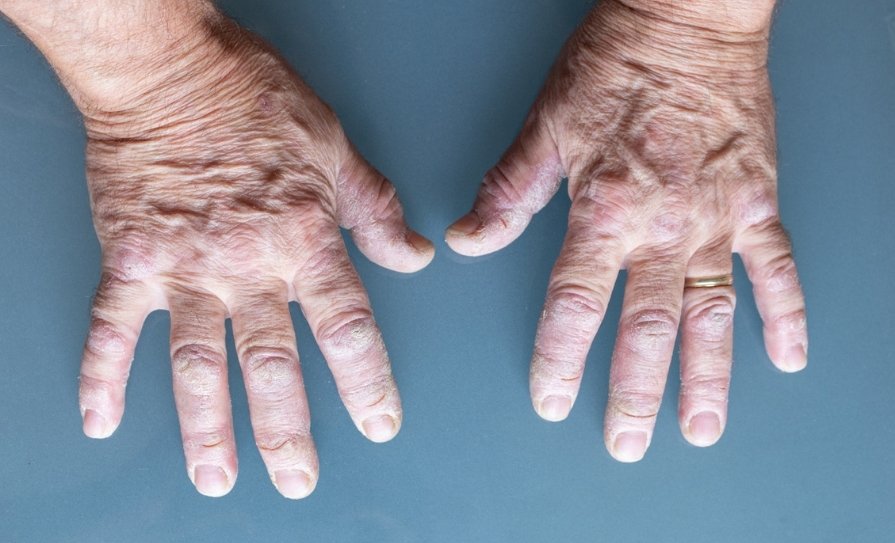
SSc is a rare connective tissue disorder with both autoimmune and vascular features. The main hallmark is tissue and vascular fibrosis that can result in clinical heterogeneity of manifestations across the skin and internal organs. This heterogeneity makes the disease variable in both the symptoms it causes and how it should be treated.
Of note, SSc has traditionally been called scleroderma, but strictly speaking this term refers only to skin involvement. Because non-skin manifestations have a significant impact, the term SSc is preferred in the EULAR recommendations.
Since its previous recommendations published in 2017, much new evidence – and new drugs – have become available for the management of SSc, thus EULAR’s treatment guidance has been reviewed and updated.
Researchers, healthcare professionals, and patients worked together to develop this new advice – including 27 people from 17 different countries. The group completed a literature review to collect up-to-date information, including findings from an online survey among people in EUSTAR – an international research network of over 200 specialist SSc centres.
The new recommendations reflect the results of several clinical trials since the last update, with the demonstration of efficacy of both synthetic and biological therapies in some patient subsets.
The new guidance, published in the 11 October 2024 issue of the Annals of the Rheumatic Diseases, include individual recommendations across nine key categories. This is a significant increase from the 16 recommendations in 2017.
Several changes in the new recommendations are significant. These include the first set of synthetic and biological targeted therapies recommended for key fibrotic manifestations of SSc, as well as first-line combination treatment for newly-diagnosed pulmonary arterial hypertension (PAH) and prioritising a new research agenda in the coming years.
The new recommendations focus on the eight key clinical/organ domains of SSc including Raynaud’s phenomenon, digital ulcers, PAH, scleroderma renal crisis, skin fibrosis, interstitial lung disease (ILD), gastrointestinal manifestations, and arthritis.
Most of the new recommendations are related to skin fibrosis and ILD. These include novel recommendations for the use of mycophenolate mofetil (MMF), nintedanib, rituximab, and tocilizumab for the treatment of these crucial disease manifestations.
The recommendations also include first-line and second-line interventions, providing increased utility for rheumatology practitioners.
Important additions to the future research agenda include consideration of novel interventions for the management of vascular, musculoskeletal and gastrointestinal manifestations, and calcinosis, as well as for the local management of digital ulcers.
A one-size-fits-all strategy cannot be implemented in SSc, where disease duration, comorbidities, patient preference, local availability, and cost of medication all need to be taken into account for informed decision-making, the EULAR document maintains.
In summary, the new recommendations note that the significant advances made in SSc vasculopathy management emphasise the treatment continuum for the use of various vasodilators and anti-remodelling drugs from Raynaud’s to digital ulcers and PAH.
Similarly, a therapeutic continuum in the interventions for skin and lung fibrosis is also apparent. These latter two domains are the ones with the most important updated information, resulting in recommendations for the use of MMF and/or rituximab, and tocilizumab for both skin and lung, and nintedanib to be used alone or in combination with MMF for the treatment of SSc-ILD.
EULAR is confident the new recommendations provide state-of-the-art guidance for physicians globally to help manage SSc patients across the spectrum of disease.
Source
Del Galdo F, et al. EULAR recommendations for the treatment of systemic sclerosis: 2023 update. Ann Rheum Dis. 2024 Oct 17:ard-2024-226430
| Organ involvement | Recommendation |
| SSc-RP |
Dihydropyridine-type calcium antagonists, usually oral nifedipine, should be used as first-line therapy for SSc-RP. PDE5 inhibitors should also be considered for treatment of SSc-RP. IV iloprost should be considered for severe SSc-RP following failure of oral therapy. |
| Digital ulcers |
PDE5 inhibitors and/or intravenous iloprost should be considered. Bosentan should be considered to reduce the number of new digital ulcers. |
| SSc-PAH |
Combination PDE5i and endothelin receptor antagonists should be considered as first-line treatment.* IV epoprostenol should be considered for advanced PAH (class III/IV). Other prostacyclin analogues or agonists can be considered. Riociguat can be considered. Anticoagulants are not recommended.* |
| Renal crisis |
Immediate use of ACE inhibitors at diagnosis of scleroderma renal crisis. Patients on glucocorticoids should have regular blood pressure monitoring to detect scleroderma renal crisis. |
| GI symptoms |
PPI should be considered for SSc-GERD and prevention of oesophageal ulcers and strictures. Prokinetics should be considered for symptomatic SSc-related motility disturbances. Rotating antibiotics should be considered for small intestinal bacterial overgrowth. |
| Skin |
Methotrexate, mycophenolate mofetil (MMF) and/or rituximab should be considered for SSc skin fibrosis.* Tocilizumab may be considered for skin fibrosis in patients with early, inflammatory dcSSc.* |
| ILD |
MMF, cyclophosphamide, or rituximab should be considered.* Nintedanib should be considered alone or in combination with MMF.* Tocilizumab should be considered.* |
| Poor prognosis | High-intensity immunosuppression (usually including cyclophosphamide) followed by autologous HSCT may be considered for selected patients with early dcSSc and poor prognosis, in the absence of advanced cardiorespiratory involvement. |
| Musculoskeletal | Methotrexate should be considered for the treatment of musculoskeletal involvement in SSc. |
Abbreviations: GERD, gastro-oesophageal reflux disease; HSCT, haematopoietic stem cell transplantation; ILD, interstitial lung disease; PAH, pulmonary artery hypertension; RP, Raynaud’s phenomenon; SSc, systemic sclerosis.