Reference: July 2025 | Issue 7 | Vol 11 | Page 7
Consider a typical clinical scenario: A 58-year-old woman presents to a heart failure clinic with rising creatinine levels and suboptimal glycaemic control. The traditional response involves multiple referrals – nephrology for kidney function and endocrinology for diabetes management, while the cardiologist focuses solely on cardiac parameters. This fragmented approach, replicated countless times across healthcare systems globally, exemplifies a fundamental flaw in contemporary chronic disease management.

This scenario underscores an emerging paradigm: These patients do not harbour three distinct diseases but rather manifest a single syndrome affecting multiple organ systems. The cardiovascular-kidney-metabolic (CKM) syndrome represents a conceptual evolution that demands immediate attention from the medical community.
The epidemiological imperative
The prevalence data for CKM syndrome reveal a public health crisis of staggering proportions. Analysis of population-based studies demonstrates that merely 10 per cent of adults in the US remain in stage 0 – completely free of CKM risk factors.1 This statistic alone should prompt serious reflection on our preventive strategies. Perhaps more concerning, longitudinal data from 2011 to 2020 show no improvement in CKM prevalence, with 90 per cent of US adults manifesting at least one component of the syndrome, and 15 per cent already in advanced stages 3-4.
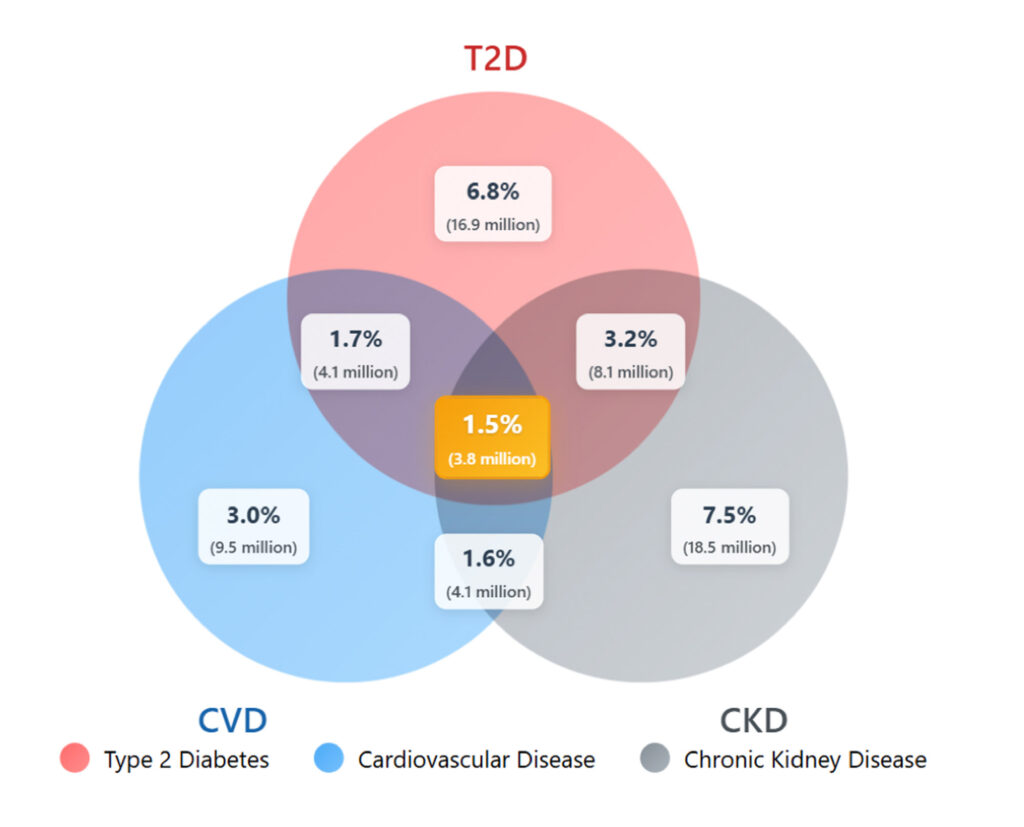
The interconnected nature of CKM components becomes strikingly apparent when examining disease overlap. Real-world data indicate that more than 25 per cent of adults have at least one CKM condition, with substantial overlap between type 2 diabetes (T2D) and chronic kidney disease (CKD) affecting 3.2 per cent of the population.2 The convergence of all three conditions – cardiovascular disease (CVD), CKD, and T2D – impacts 1.5 per cent of adults, representing nearly 4 million individuals in the US alone (Figure 1).
These epidemiological patterns extend to clinical trial populations, providing crucial context for interpreting research findings. Analysis of the FINE-HEART programme, encompassing the FIDELIO-DKD and FIGARO-DKD trials and the FINEARTS-HF study, reveals that approximately 90 per cent of enrolled participants exhibited overlapping CKM conditions (Figure 2).3 This high prevalence suggests that our evidence base inherently applies to patients with multisystem involvement, challenging the notion of single-disease therapeutic approaches.
The CKM staging system: A framework for comprehensive risk assessment
The American Heart Association Presidential Advisory recently introduced a staging system that fundamentally reconceptualises cardiovascular and metabolic risk (Figure 3).4 This framework, progressing from stage 0 through stage 4, provides structure for understanding disease evolution and targeting interventions.
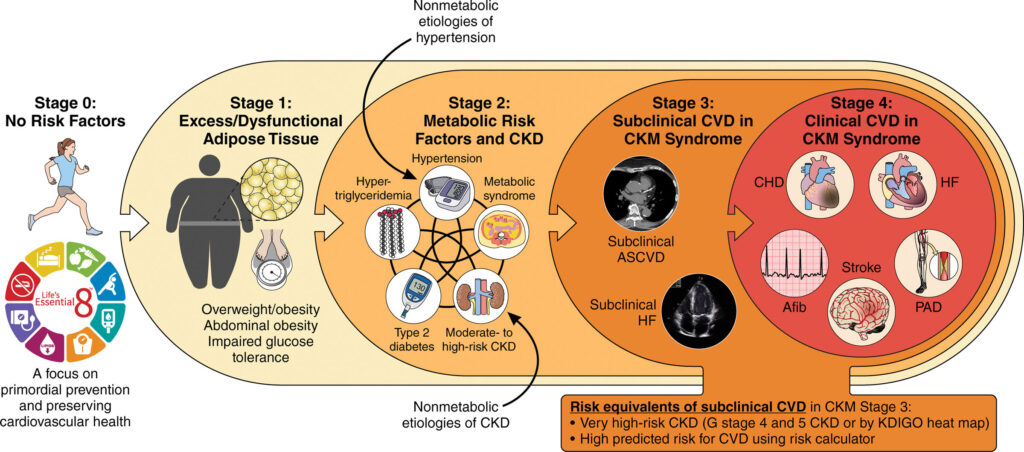
Stage 0 represents optimal cardiovascular-metabolic health and is characterised by the absence of risk factors. The explicit inclusion of this stage serves a critical purpose – emphasising primordial prevention. The fact that only 10 per cent of adults remain in this stage highlights the urgent need for population-level preventive strategies.
Stage 1 identifies individuals with excess or dysfunctional adipose tissue without additional metabolic risk factors. This classification recognises adiposity, particularly visceral adiposity, as a pathophysiological state rather than merely a risk marker.
The stage acknowledges important ethnic variations in adipose tissue distribution and metabolic risk. Asian populations, for instance, demonstrate higher visceral adiposity at equivalent body mass indices compared to Caucasian populations, necessitating adjusted obesity definitions and heightened vigilance for metabolic dysfunction at lower body weights.5
Stage 2 encompasses the emergence of metabolic risk factors including hypertension, dyslipidaemia, early CKD, and hyperglycaemia. The staging system innovatively incorporates both metabolic and non-metabolic pathways to disease, recognising that conditions such as hypertensive nephropathy or genetic kidney diseases can initiate the CKM cascade through alternative mechanisms.
Stage 3 represents subclinical target organ damage, including pre-heart failure (stage B in traditional classification), subclinical atherosclerosis, or stage 4 or 5 CKD. This stage identifies a critical intervention window where organ damage has occurred, but clinical symptoms have not yet manifested.
Stage 4 encompasses clinically manifest disease across the CKM spectrum, characterised by the convergence of symptomatic heart failure, established coronary and peripheral artery disease, and progressive cardiac electrical remodelling that predisposes to atrial fibrillation and subsequent stroke risk.

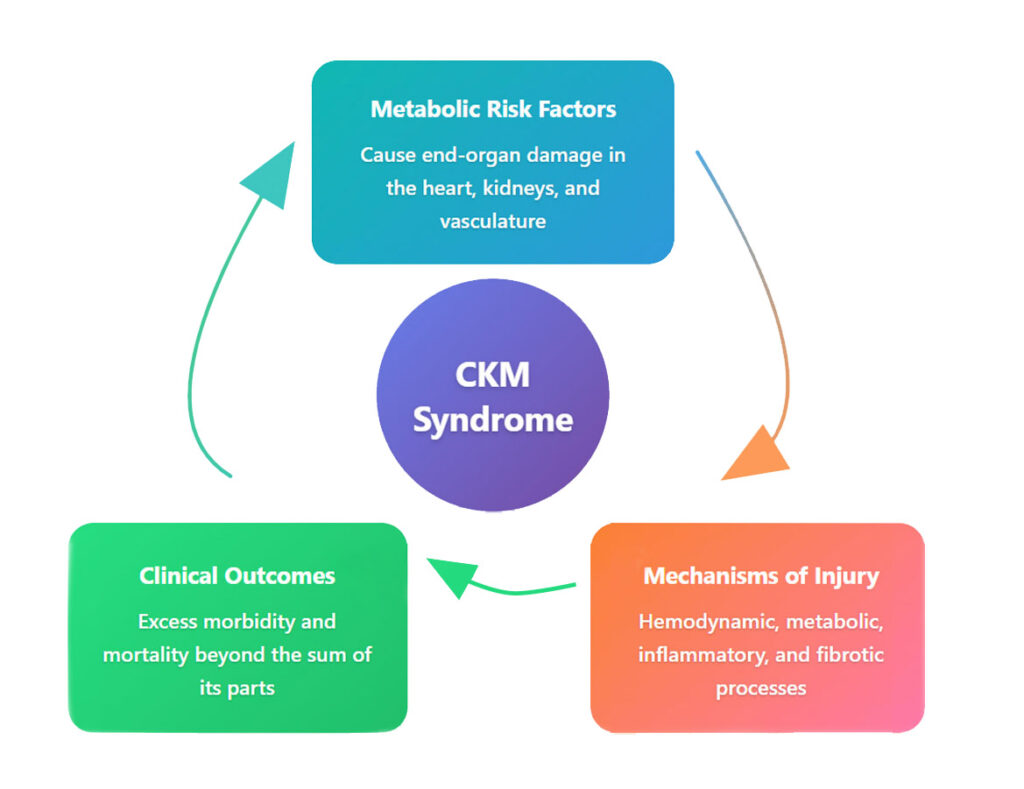
Pathophysiological integration: Understanding the vicious cycles
The pathophysiology of CKM syndrome involves complex bidirectional relationships that amplify disease progression (Figure 4). CKD serves as a particularly potent cardiovascular risk multiplier through several mechanisms.6
The uraemic milieu creates a pro-inflammatory state that accelerates atherosclerosis and myocardial dysfunction. Retained uraemic toxins, oxidative stress, and chronic inflammation establish conditions favourable for vascular injury and cardiac remodelling.7 Furthermore, the disturbed mineral metabolism characteristic of CKD – hyperphosphataemia, secondary hyperparathyroidism, and vitamin D deficiency – promotes vascular calcification, increasing arterial stiffness and cardiac afterload.8
The neurohormonal activation accompanying cardiorenal dysfunction creates self-perpetuating cycles of organ damage (Figure 5). Reduced cardiac output triggers renal hypoperfusion, activating the renin-angiotensin-aldosterone system and sympathetic nervous system. While initially compensatory, chronic neurohormonal activation promotes sodium retention, vasoconstriction, and progressive fibrosis in both cardiac and renal tissues.
Metabolic dysfunction contributes additional pathophysiological complexity. Adipose tissue, particularly visceral adipose tissue, functions as an endocrine organ secreting pro-inflammatory cytokines and adipokines that promote insulin resistance, endothelial dysfunction, and myocardial fibrosis. This inflammatory state synergises with the uraemic milieu and neurohormonal activation to accelerate organ damage.
Anaemia represents an under-appreciated, but critical component of CKM pathophysiology. The multifactorial anaemia of CKD – resulting from decreased erythropoietin production, uraemic inhibition of erythropoiesis, and reduced red blood cell survival – exacerbates cardiac workload by necessitating increased cardiac output to maintain tissue oxygenation. This increased haemodynamic burden further stresses the failing heart, exemplifying the interconnected nature of CKM syndrome.
Reconceptualising heart failure within the CKM framework
The integration of CKM concepts necessitates re-evaluation of traditional heart failure staging and diagnosis.9 Current guidelines now recognise CKD as a risk factor for heart failure (stage A), though previous guidelines did not. However, this relationship requires more nuanced consideration.
Advanced CKD almost invariably associates with subclinical cardiac structural changes, including left ventricular hypertrophy, diastolic dysfunction, and myocardial fibrosis. These findings suggest that patients with advanced CKD may warrant classification as pre-heart failure (stage B).
Diagnostic challenges emerge when evaluating heart failure in CKD patients. The overlapping symptomatology like dyspnoea, fatigue, and peripheral oedema complicates clinical assessment. Both conditions elevate natriuretic peptides, though CKD increases these biomarkers independent of cardiac filling pressures through decreased renal clearance and increased cardiac stress. Current recommendations suggest adjusted thresholds for ruling in heart failure in CKD patients, though optimal cutoff values remain debated.
The diagnostic approach in CKM syndrome requires integration of multiple assessment modalities. While non-elevated natriuretic peptides retain high negative predictive value, elevated levels require correlation with imaging findings and clinical presentation.
Echocardiography provides structural and functional assessment, though image quality may be compromised in volume-overloaded states. In complex cases, invasive haemodynamic assessment, potentially with provocative manoeuvres, may be necessary to establish diagnoses and guide management.
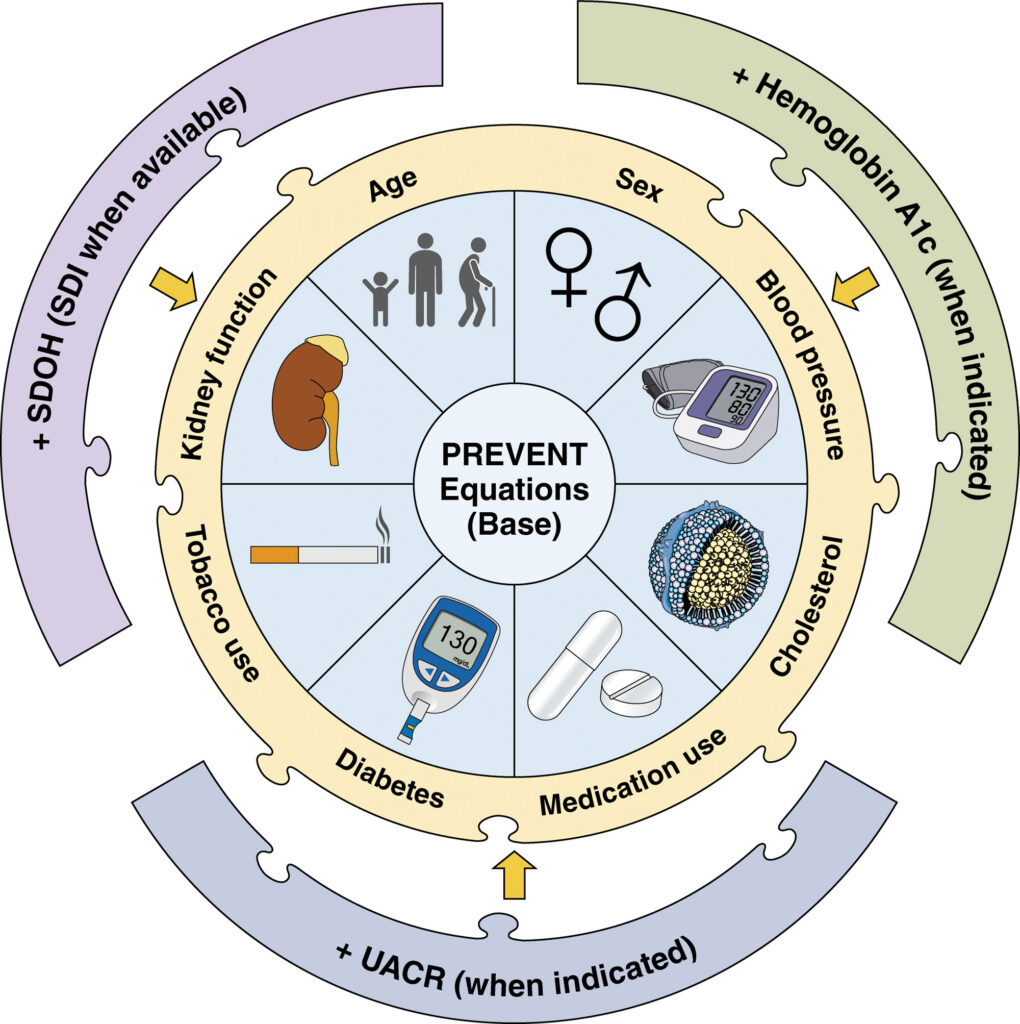
The PREVENT equations: Comprehensive risk assessment for the CKM era
The development of the PREVENT equations represents a fundamental advance in cardiovascular risk assessment aligned with CKM syndrome concepts (Figure 6).10 Unlike previous tools that focused primarily on atherosclerotic events, PREVENT calculates total CVD risk, explicitly including heart failure – a recognition of its growing importance as a cardiovascular endpoint.
Key innovations in the PREVENT equations reflect evolving understanding of cardiovascular risk determinants:
- Integration of kidney function: The inclusion of estimated glomerular filtration rate (eGFR) as a core predictor acknowledges CKD’s critical role in cardiovascular risk stratification.
- Elimination of race-based coefficients: Removing race as a predictor addresses concerns about biological validity and potential contributions to healthcare disparities, aligning with recent modifications to kidney function equations.
- Sex-specific risk estimation: Development of separate equations for men and women acknowledges important differences in cardiovascular risk factors and disease manifestations.
- Flexible risk refinement: Optional inclusion of urine albumin to creatinine ratio (UACR), haemoglobin A1c, and social determinants of health (SDOH) indices allows refined risk assessment when comprehensive data are available.
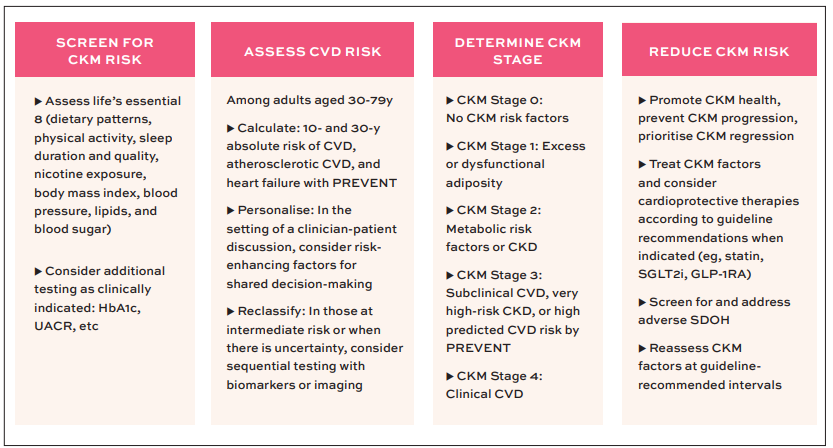
The clinical implementation pathway follows a systematic approach (Figure 7): Screening for CKM risk factors, quantifying risk using PREVENT equations, determining CKM stage, and implementing stage-appropriate interventions aimed at preventing progression or achieving regression.
The Imperative for UACR assessment
A critical gap in current practice involves the underutilisation of UACR measurement, particularly in cardiology settings. The Kidney Disease Improving Global Outcomes (KDIGO) classification system emphasises dual assessment of kidney function through eGFR and UACR, recognising that albuminuria independently predicts cardiovascular and kidney outcomes. Patients may maintain normal eGFR while manifesting significant albuminuria, representing both increased risk and therapeutic opportunity, as many cardio-renal protective therapies specifically reduce albuminuria.
The failure to routinely assess UACR in heart failure patients represents a missed opportunity for comprehensive risk stratification and targeted intervention. Integration of UACR measurement into routine cardiovascular assessment protocols is essential for optimal CKM syndrome management.
Therapeutic integration: Multi-organ protection strategies
The therapeutic landscape for CKM syndrome has evolved dramatically with the emergence of medications providing multi-organ protection. This evolution challenges traditional organ-specific prescribing patterns and demands a more integrated approach.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors, initially developed as glucose-lowering agents, demonstrate remarkable cardio-renal protection across the CKM spectrum, reducing heart failure hospitalisations, and slowing CKD progression independent of glycaemic effects.
Glucagon-like peptide-1 (GLP-1) receptor agonists provide metabolic benefits while reducing major adverse cardiovascular events, offering particular value in patients with obesity and/or T2D. Non-steroidal mineralocorticoid receptor antagonists, exemplified by finerenone, reduce both cardiovascular and kidney outcomes in diabetic kidney disease, addressing inflammatory and fibrotic pathways central to CKM pathophysiology.
The selection and combination of these agents should reflect individual patient phenotypes and CKM stage rather than arbitrary specialty boundaries. Optimal management requires moving beyond siloed prescribing toward integrated therapeutic strategies addressing multiple pathophysiological mechanisms simultaneously.
Conclusion: A call for integrated action
The CKM syndrome represents more than semantic evolution; it embodies a fundamental shift in understanding chronic disease in the 21st Century. The epidemiological reality, with 90 per cent of adults manifesting CKM components and millions suffering from multi-organ involvement, demands immediate action.
The scientific foundation is robust. Pathophysiological understanding reveals intricate connections between cardiovascular, kidney, and metabolic systems. Diagnostic tools like the PREVENT equations enable comprehensive risk assessment. Therapeutic options providing multi-organ protection are available and proven effective. What remains is translation of this knowledge into clinical practice.
The transition from siloed to integrated care requires commitment across multiple stakeholders. Clinicians must expand their perspective beyond organ-specific management, embracing comprehensive CKM assessment and treatment. Healthcare systems must restructure care delivery to facilitate integration. Policy-makers must align incentives with optimal CKM care. With the framework established and tools available, the opportunity to transform chronic disease management is within reach.
References
- Aggarwal R, Ostrominski JW, Vaduganathan M. Prevalence of cardiovascular-kidney-metabolic syndrome stages in US adults, 2011-2020. JAMA. 2024;331(21):1858-1860.
- Ostrominski JW, Arnold SV, Butler J, et al. Prevalence and overlap of cardiac, renal, and metabolic conditions in US adults, 1999-2020. JAMA Cardiol 2023;8(11):1050-60.
- Vaduganathan M, Filippatos G, Claggett BL, et al. Finerenone in heart failure and chronic kidney disease with type 2 diabetes: FINE-HEART pooled analysis of cardiovascular, kidney, and mortality outcomes [published correction appears in Nat Med. 2024;30(12):3778.
- Ndumele CE, Rangaswami J, Chow SL, et al. Cardiovascular-kidney-metabolic health: A Presidential advisory from the American Heart Association. Circulation. 2023;148(20):1606-1635.
- Lim U, Ernst T, Buchthal SD, et al. Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index. Nutr Diabetes. 2011;1(5):e6.
- Ndumele CE, Neeland IJ, Tuttle KR, et al. A synopsis of the evidence for the science and clinical management of cardiovascular-kidney-metabolic (CKM) syndrome: A Scientific Statement from the American Heart Association. Circulation. 2023;148(20):1636-1664.
- Lekawanvijit S. Cardiotoxicity of uremic toxins: A driver of cardiorenal syndrome. Toxins (Basel). 2018;10(9)352.
- Dube P, DeRiso A, Patel M, et al. Vascular calcification in chronic kidney disease: Diversity in the vessel wall. Biomedicines. 2021;9(4):404.
- Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23(3):352-380.
- Khan SS, Coresh J, Pencina MJ, et al. Novel prediction equations for absolute risk assessment of total cardiovascular disease incorporating cardiovascular-kidney-metabolic health: A Scientific Statement from the American Heart Association. Circulation. 2023;148(24):1982-2004.