Blood transfusion is ‘one of the most frequently used therapies worldwide and is associated with benefits, risks and costs’(Frankfurt PBM Consensus Conference, 2018). The World health Organisation (WHO), in March 2020, published an interim guidance on maintaining a safe and sustainable blood supply during the pandemic outbreak of SARS-CoV-2 (Covid-19), noting the potential for reduction in supply of blood and blood components and adverse impact on blood system activities (and a theoretical, but likely minimal, risk of transfusion transmission).
At the same time the European Centre for Disease Prevention and Control (ECDC) provided management options for safe and sustainable supply of substances of human origin (SoHO). The newly formed National Transfusion Advisory Group (NTAG), with a brief for safe and sustainable supply, initially focused on shortage preparedness planning and agreed to advance patient blood management (PBM) as an appropriate medium and long term strategy.
Lee et al in 2017 (Transfusion 56, 1347-58) assessed the six year Western Australia PBM initiative and showed a reduction in transfusion together with a decrease pre-operative anaemia and length of stay.
The NTAG was also concerned at the halt to the trend of year-on-year reduction in demand for blood transfusion to 2016 (11 per cent over 5 years) and indeed a small increase since (3 per cent), similar to but less pronounced than the UK trend. While additional demand may arise from demographic trends (higher transfusion rates in older patients) and medical advances (longer transfusion dependency for haemato-oncology patients) no national data is available to confirm appropriate clinical application.
Furthermore, the NTAG national survey March 2020 across the 45 hospitals with transfusion laboratories, identified a higher than expected transfusion rate of 2.1 red cell components per patient but this might reflect the selective patient cohorts who required in-patient care at that time.
Development of patient blood management -PBM
While PL Mollison in 1993 advocated investigation of pre-operative anaemia, differing transfusion thresholds relating to clinical conditions and cautioned against repeated blood sampling, it took a further decade for the concept of patient blood management (PBM) to come to the fore. PBM is considered “the timely use of evidence based medical and surgical concepts designed to maintain haemoglobin concentration, optimise haemostasis and minimise blood loss” (Society for the Advancement of Blood Management, Professional Definition Patient Blood Management, Sept 2012).
This aims to improve the care of patients who may need transfusion in an effort to improve patient outcomes. Three core pillars are described for PBM – optimise red cell mass, minimise blood loss and bleeding, harness and optimise physiological reserve of anaemia. Evidence-based use of platelets and plasma and alternatives to transfusion are key elements.
A focus on clinical governance and healthcare improvement science together with surgical technical developments (including minimally invasive surgery) were pivotal in developing interest in PBM. Landmark
publications include the TRICC study – non-inferior restricted transfusion thresholds for patients in critical care (Herbet, 1999 NEJM), the Austrian benchmark study (Gombotz et al, Transfusion, 2007) and later the Cochrane systematic review 2016, where Carson evidenced the safety of a restrictive transfusion policy.
The Society for the Advancement of Blood Management advocated patient centred decisions, managing anaemia, inter-disciplinary blood conservation modalities and optimising coagulation. In 2001 the Australian National Blood Authority (NBA) Clinical Advisory Council advised replacement of product focused guidelines with patient focused guidelines and by 2006 noted that PBM was an excellent clinical framework for these.
By 2010 a WHO PBM concept paper was issued with a focus on preventing conditions that might need blood transfusion, appropriate diagnosis of anaemia and optimal treatment, use of alternatives, blood conservation, and good surgical and anaesthetic techniques.
In the past decade, the Australian NBA established a jurisdictional blood committee (JBC) working group which developed six PBM modules between 2011 and 2016 (Module 1 Critical bleeding/Massive transfusion: Module 2 Peri-operative: Module 3 Medical: Module 4 Critical Care: Module 5 Obstetrics and Maternity: Module 6 Neonatal and Paediatric). As a collaborative initiative the UK National Blood Transfusion Committee and the National Health Service Blood and Transplant (NHSBT) adopted the PBM concept in 2012.
In 2016, PBM was adopted by the European Union and the American Association of Blood Banks (AABB). At the same time, the WHO strengthened its position with a recommendation to establish/strengthen systems for safe and rational use of blood products, to provide training for all staff involved in clinical transfusion… and to promote the availability of transfusion alternatives and patient blood management [WHA 63.12 (6)]. PBM as a concept is adopted in the International Choosing Wisely Initiative.
The approach to implementation in the UK and Australia is instructive
After a 2013 survey of readiness in 149 NHS Trusts PBM recommendations were launched in 2014. Less than 50 per cent of Trusts were actively managing anaemia and only 27 per cent had implemented restrictive thresholds. The NHSBT PBM tool kit was developed with a focus on single unit transfusion.
The 2015 national comparative audit showed 20 per cent inappropriate use of red cells and 20-30 per cent in relation to platelets and plasma. A five-point anaemia plan was launched in 2016 supported by PBM teams delivering an ongoing programme of support, education, audit, research and specialist advice to ensure a co-ordinated approach to improving transfusion practice, regionally and nationally. A special requirements app was launched.
In Australia, governance lies with the JBC and NBA clinical advisory committee. Their strategy is to facilitate activities and develop materials at a national level that support implementation at a health provider level with a suite of tools for local customisation. PBM tools include a national reference set of best practice guidelines and education and training (including blood safety eLearning Australia programme), promotion and communication (through website and networking) and incorporating key data sets into the ITC strategy.
Current International Guidelines
The AABB PBM standards require institutional support (including budget support) for an active programme with performance metrics and clinician feedback. Treatment of massive blood loss and appropriate inventory management form part of these standards. The UK’s National Institute of Clinical Excellence (NICE) set out PBM quality standards in 2016.
Four statements focus on research, identification of iron deficiency anaemia and access to iron supplementation, use of tranexamic acid for adults undergoing surgery with expected moderate blood loss, requirement to clinically re-assess and check haemoglobin levels after each unit of red blood cells transfused for appropriate patients and provision of verbal and written information for patients who may need transfusion.
The 2018 Frankfurt Consensus Conference agreed ten clinical recommendations and twelve research recommendations which refer to pre-operative anaemia, red blood cell transfusion thresholds for adults and implementation of PBM programmes.
Developments in Ireland
With the threat to the sustainability of the blood supply from vCJD associated donor deferrals, Micheál Martin TD, then Minister for Health and Children, set up the Irish National Blood Strategy Group in 2001.This group was tasked to support the development of best practice in blood utilisation, blood stock management and the implementation of guidelines. They undertook an inventory snapshot, peri-operative audit and a blood utilisation survey.
These informed recommendations which included the establishment of pre-surgical assessment units and structures – National, Regional and Hospital, supported by a Consultant Haematologist in Transfusion Medicine. It was recommended that the national body should prepare clinical guidelines and monitor practice through audits at agreed intervals. Support from procuring a blood order system prompting clinicians and ongoing continuous professional development for all clinical staff in blood use decision making was also recommended.
In the interim the 45 hospital-based transfusion laboratories in Ireland have been accredited to the mandatory standard ISO 15189 and AML-BB and the HSE has a blood stock unit to optimise inventory distribution.
A Clinical Lead Advisor for Transfusion was appointed in September 2019 and the National Transfusion Advisory Group (NTAG) held its inaugural meeting at the RCPI in January 2020.
NTAG
The NTAG is a representative group of clinical, laboratory, allied health professionals, national bodies including haemovigilance, patients, donor, and service users engaged with transfusion services in Ireland. NTAG provides a national framework for consideration of transfusion matters and guideline development in relation to transfusion practice and associated implementation accessibility pathways.
Dr Michael Dockery, Consultant Anaesthesiologist, University Hospital Waterford, chairs the NTAG with Dr David Menzies Consultant in Emergency Medicine, St Vincent’s University Hospital (SVUH), Deputy Chair. NTAG is supported by three ‘Pillar’ societies whose members are engaged in transfusion practice on a day to day basis-the Irish Haematology Society transfusion special interest group, the Academy of Clinical Science and Laboratory Medicine (ACSLM) NTAG Scientific Committee and the National Haemovigilance Special Interest Group.
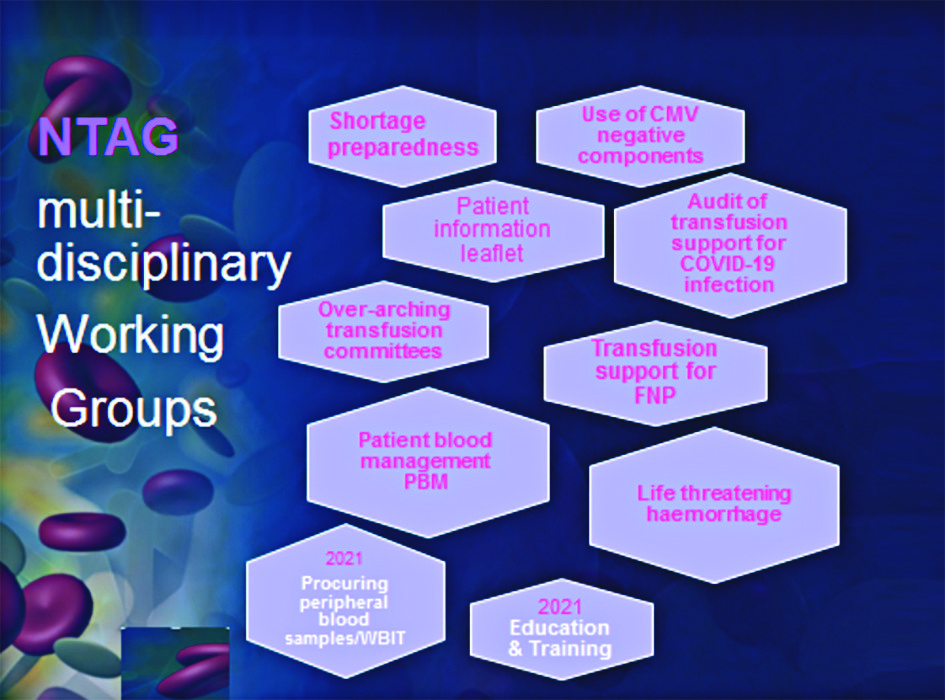
With the declaration of the pandemic, the NTAG prioritised blood supply shortage preparedness and the CLA made available three guidance documents to hospital transfusion teams by mid-March last year. A multidisciplinary working group was established to generalise these guidelines to threats to the sustainability of the blood supply from any source and address specific aspects of implementation. Since then the NTAG set up additional working groups supporting PBM (see Figure 1).
NTAG PBM working group
The NTAG PBM working group (Chair Dr Joan Fitzgerald) is assisted by other NTAG working groups addressing: transfusion support of foetus, neonates and paediatric patients, patient information leaflet, and life-threatening haemorrhage. In addition, a Ministerial commissioned NCEC guideline development group is addressing intra-operative acute life threatening haemorrhage.
From these groups, patients in Ireland should be well supported with PBM guidelines. However, for sustainable practice enhancement further steps are essential and can be informed by international experience. These include implementation within a cohesive national framework, planned with local champions, programmes to address education and training needs, recording and collection of well-defined consistent data items of high quality and definition of key performance indicators.

A national audit conducted on a periodic basis (to consider the structural, process and output aspects of PBM management), with the appropriate use of transfusion support at its core, will provide evidence of practice enhancements and gaps in the equity of access to PBM for patients within Irish healthcare systems.
National Transfusion Advisory Group NTAG
Governance
Dr Joan Fitzgerald, Consultant Haematologist Chair NTAG WG: PBM
Dr Joan Power, Clinical lead for Transfusion
Prof Stephen Field, MSD IBTS
Dr Michael Dockery, Chair NTAG
Working Group Scope – Draft 1 Objective
To develop a stakeholder proposed NTAG Guideline for Patient Blood Management. Interim guidance may be issued. The NTAG PBM Guideline will be a controlled document(s) and the review process will be established. The Guideline will be authorised by the NTAG WG Chair, the Clinical Lead Advisor, Medical & Scientific Director IBTS and Chair NTAG.
This editorial has been sponsored by Vifor Pharma UK Ltd. Commissioned by the Medical Independent and independently written, Vifor Pharma UK Ltd has had input in to the topic, suggested authors, and performed a pre-publication review for compliance purposes.







Leave a Reply
You must be logged in to post a comment.