An overview of the evidence on the role of exercise as a non-pharmacological therapeutic treatment for non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is now the leading cause of chronic liver disease worldwide, linked to the increasing global incidence of type 2 diabetes mellitus (T2DM) and obesity. NAFLD is increasingly becoming the leading cause of liver cirrhosis, liver cancer and liver transplantation worldwide. Patients with NAFLD are at a high risk of cardiometabolic comorbidities, including central obesity, insulin resistance and cardiovascular disease (CVD), which predispose patients to metabolic syndrome. In fact, NAFLD is argued to be the hepatic manifestation of the metabolic syndrome. The identification of cardiometabolic comorbidities in NAFLD patients is important, as CVD is the leading cause of mortality in this population.
NAFLD comprises a spectrum of disease that ranges from simple steatosis, to the more severe inflammatory subtype: Non-alcoholic steatohepatitis (NASH). Approximately 17-to-25 per cent of patients with NAFLD will have NASH, and 7 per cent of patients with NASH will develop cirrhosis. The gold standard for the diagnosis of NAFLD is a liver biopsy, which identifies the degree of hepatic steatosis, as well as liver-cell injury and inflammation.
Non-invasive assessments such as imaging and algorithms based on biochemical tests may also identify features of NAFLD and highlight patients who require a biopsy.
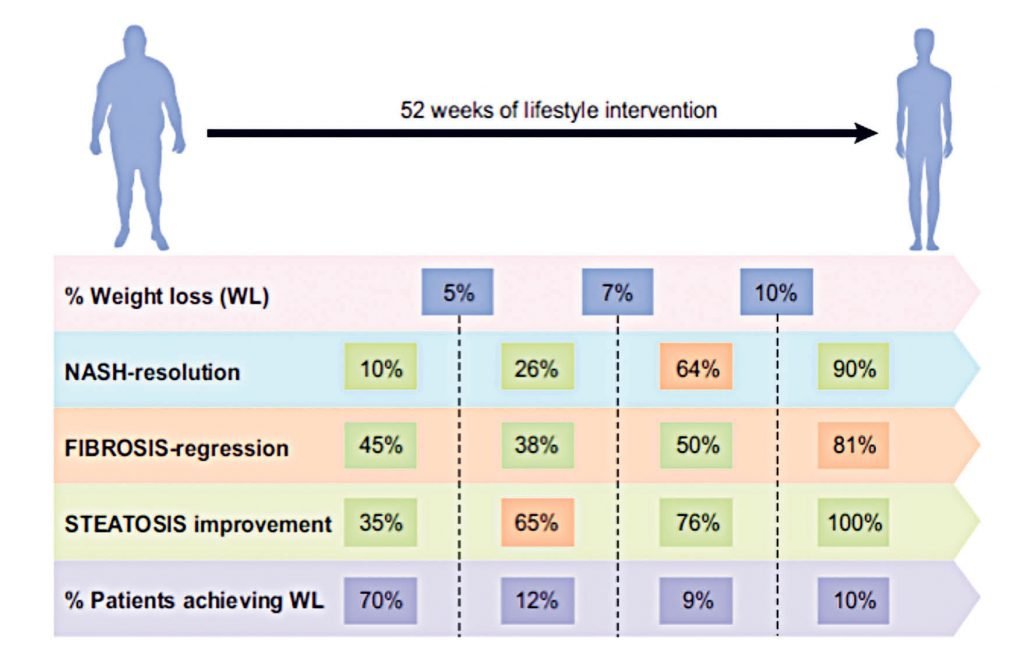
To date, there are no approved pharmacological therapies for treating NAFLD and consequently, lifestyle interventions remain the cornerstone of treatment, with current EASL guidelines recommending a target weight loss of 7-to-10 per cent to facilitate regression of NASH and fibrosis. These recommendations are based on evidence such as the 2015 study by Vilar-Gomez and colleagues, which incorporated a 52-week lifestyle intervention (combined diet and exercise) using paired liver biopsies in 293 patients, with a focus on weight-loss. The authors reported significant histological improvements in both NASH and fibrosis staging that were related to weight loss, and the extent of the histological benefits was dependent on the degree of weight loss, with ≥10 per cent weight loss leading to an almost complete regression of NASH, steatosis and fibrosis (Figure 1). Despite weight loss having significant therapeutic benefit, patients with NAFLD struggle to meet or maintain weight loss longitudinally. In the study by Vilar-Gomez, et al, only 10 per cent of patients achieved 10 per cent weight loss (Figure 1).
The role of exercise as a non-pharmacological therapeutic treatment for NAFLD is also worth exploring. Physical activity (PA) and exercise have been consistently demonstrated to result in favourable health outcomes for all ages and ethnicities.
The World Health Organisation (WHO) and American College of Sports Medicine recommend that all adults perform at least 150 minutes of moderate-to-vigorous PA per week for health. However, patients with NAFLD are reported to participate in less PA and more sedentary behaviour compared to matched controls. Until recently, lifestyle intervention advice for NAFLD patients took the form of combined dietary and exercise modifications, and the independent role of exercise was unclear. However, in 2017, a meta-analysis of patients with established NAFLD identified 24 exercise therapy (ET) studies (both aerobic and resistance exercise), indicating the increase in exercise studies being conducted in this area, and concluded that the predominant end-point in ET studies was the impact of ET on hepatic fat, both volume and content. Researchers reported that both resistance and aerobic exercise — without significant weight loss — results in a 20-to-30 per cent reduction in hepatic fat, as assessed by non-invasive methodologies, such as MRI and transient elastography (FibroScan). The proposed mechanisms responsible for reduction in liver fat include exercise-induced changes in energy balance, circulatory lipids and insulin sensitivity, and additionally, changes in visceral adiposity and cardiovascular health (Figure 2).
Despite the increased research in this area, the impact of ET on histological end-points such as fibrosis in NAFLD remains unclear. The only exercise-only (ie, no planned or targeted dietary intervention) study that used histological end-points as outcome measures reported no significant histological changes following a resistance exercise intervention. This study design was a six-month, low-intensity training programme (50 per cent of one-repetition maximum), with nine patients in the exercise group completing the intervention, with no subsequent histological benefits that reached statistical significance.
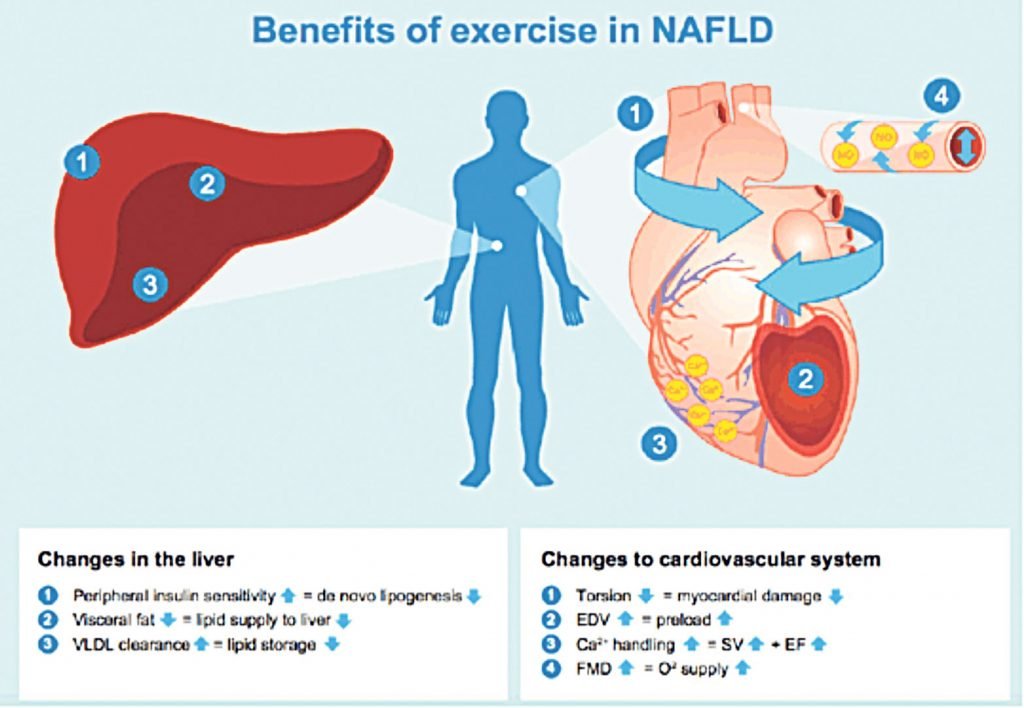
adapted from Romero-Gomez, et al
Firstly, the sample size may have been too small to detect a significant change in histological end-points, and secondly, only patients without T2DM were recruited for this study, which may be a misrepresentation of the larger NAFLD cohort seen in most hepatology clinics. Furthermore, resistance exercise at the prescribed intensity and frequency may not have been of a sufficient exercise load to induce intrahepatic improvements. Although the optimal dose, frequency and type of exercise for improving features of NASH and fibrosis remains unknown, cross-sectional studies report that moderate-to-vigorous intensity PA is required. Consequently, there is currently no evidence to suggest that weight loss can improve features of NASH beyond steatosis. There is therefore a need for more trials which assess the independent effects of exercise on features of NASH and fibrosis using histological end-points.
Cardiorespiratory fitness
The role of cardio-respiratory fitness (CRF) as a marker of liver health is an emerging area of research interest. CRF is a component of physical fitness and is the physiological manifestation of aerobic exercise participation. Compared to resistance exercise, aerobic exercise results in relatively higher energy consumption and improves CRF. Poor CRF and insufficient PA are major public health problems associated with increased mortality and morbidity. CRF has also been validated as an independent predictor of all-cause mortality in NAFLD populations and therefore represents an important clinical end-point for these patients. A higher CRF has also been demonstrated to better improve NAFLD patients’ responsiveness to lifestyle interventions, as well as being an important factor for weight-loss maintenance. In NAFLD studies to date where CRF has been assessed, studies have focused on the relationship between CRF and hepatic fat content, and the relationship between CRF, NASH and steatofibrosis thus remains largely unknown.
In 2008, researchers reported that patients with more severe liver injury (NAFLD Activity Score (NAS) ≥ 5) had significantly lower CRF compared to patients with less-severe liver injury (NAS <5).
In our study investigating the role of CRF during aerobic ET in 16 NAFLD patients, presented at the European Association for the Study of the Liver (EASL) 2019 meeting, we demonstrated that 58 per cent and 67 per cent of patients regressed one fibrosis stage and one hepatocyte ballooning stage, respectively, following a 12-week aerobic exercise intervention. Improvements in fibrosis and hepatocyte ballooning were significantly associated with improvements in CRF, strengthening the importance of CRF as a clinical outcome for NAFLD patients.
This pilot study paves the way for larger, randomised controlled trials to investigate the effects of aerobic exercise on histological features of NAFLD.
While there is no argument that exercise is an effective lifestyle modification for patients with NAFLD, the biggest challenge for patients and healthcare providers, however, is the long-term integration of exercise within daily life in the unsupervised setting. As reported by Blair, physical inactivity is the biggest public health problem of the 21st Century. A meta-analysis of diet and exercise intervention for weight loss in 2009, and more recently the Diabetes Prevention Study, highlighted the challenges of sustaining ET in patients who would benefit from lifestyle interventions. Following a 16-week supervised exercise trial in patients with NAFLD with documented improvements in liver fat and CRF, Pugh and colleagues reported that the benefits were not sustained when patients were reassessed at 12-months, concluding that effective mechanisms for promoting the long-term sustainability of exercise in NAFLD cohorts are urgently required.
The role of eHealth
One potential way to facilitate the transition of exercise into a community setting may be the use of smart technology, such as wearable devices and eHealth intervention frameworks. eHealth provides easier and quicker access to information regarding PA promotion, is less resource-intensive and allows for the prescription of PA to patients who may not normally come in contact with face-to-face interventions. Multiple reviews have reported the benefits of using eHealth interventions for the promotion and maintenance of PA in both older populations and chronic disease populations. Studies investigating the use of smart technology for the prescription of exercise in NAFLD cohorts are limited, but studies are beginning to emerge with promising findings, which may enable better integration of ET into the community setting. In two recent ET studies using an eight-week, web-based, exercise intervention in a group of 44 patients with biopsy-proven NAFLD, significant improvements in CRF and surrogate measures of hepatic steatosis and fibrosis were reported. Importantly, these benefits were sustained at 20-week follow-up assessments. The authors highlighted that the individualised nature of the programme, which was tailored to the patient’s baseline CRF and daily routines, was imperative to its successful implementation and resulted in a very low dropout rate and continued self-driven exercise. The authors also recommend that guidance from each individual patient should be sought when developing an individualised exercise intervention, to ensure it is feasible at the individual level. To date, there are no studies which have investigated the feasibility of using wearable technology to promote PA adherence in an NAFLD cohort. This approach may provide patients with a more convenient approach to increasing PA participation and should be investigated in future studies.
Barriers and motivators
Another approach to the challenge of improving the long-term sustainability of exercise is to identify the determinants of PA participation. Understanding the barriers and motivators to engaging in PA will help to develop interventions that maximise PA participation. By identifying the determinants of PA participation, important insights may be gained, which will help highlight modifiable factors that may enable greater PA adherence longitudinally in patients with NAFLD. In 2010, Frith and colleagues reported that patients with NAFLD understand the benefits of exercise but lack the confidence to undertake it. Further studies have reported that fatigue and PA were inversely associated in a group of patients with biopsy-proven NAFLD, suggesting that fatigue may be an important barrier to engaging in PA in this population. The identification and elimination of these barriers at the individual level are imperative to successful longitudinal adherence to PA in this population. There are many techniques and behaviour models that can be employed which have been proven to increase PA participation and that may provide patients with NAFLD with the appropriate skills to maintain PA participation longitudinally. The use of motivational interviewing (MI) alongside behaviour change models, such as the transtheoretical model, the stages of change readiness model and the self-regulation theory, have demonstrated success as a pre-screening tool to motivate patients to become more physically active. The meta-analysis by O’Halloran et al concluded that incorporating MI into the ‘standard of care’ model has good potential to induce modest improvements in PA in many chronic disease populations. The use of MI in conjunction with the use of evidence-based, behavioural change models may enhance an NAFLD patient’s self-efficacy to exercise — this has been cited as a strong intrinsic motivator to exercise participation.
Future approaches
A major challenge with ET as a treatment for NAFLD patients is its long-term sustainability. At the individual level, the identification and modification of the determinants of PA participation will tailor PA interventions in order to maximise long-term participation. One approach could involve the creation of PA referral centres with experienced exercise professionals, with clinicians in primary care centres referring patients to exercise specialists. Referral schemes for weight loss in the UK have been successful at reducing body mass by >5 per cent within 12 weeks. A similar model for promoting PA and treating CRF as a ‘Clinical Vital Sign’ may represent the next step for the management of patients with NAFLD (Figure 3). Furthermore, eHealth and wearable technology interventions, deployed at an individual level, have huge potential to promote community-based exercise maintenance in NAFLD cohorts. Future studies should investigate the feasibility of a systems approach to promoting long-term adherence to ET for NAFLD cohorts, other chronic disease cohorts, and for long-term general health in line with the national ‘Healthy Ireland’ strategy for improving PA in Ireland.
References on request












Leave a Reply
You must be logged in to post a comment.