Small fibre peripheral neuropathy (SFN) is recognised as an expanding public health problem with 40 million individuals identified in the US. Painful SFN is characterised by structural injury selectively affecting small diameter sensory and/or autonomic axons dominated by pain. Diabetic neuropathy is the most common peripheral neuropathy in the US and globally.
With the prevalence of diabetes in adults rising to 10.8 per cent in the US and 4.3 per cent in the UK, improving the management of peripheral neuropathy has never been more important. Unfortunately the pathogenesis of injury to small nerve fibres is not well understood and therefore treatment can prove challenging.
This overview seeks to highlight some of the causes of SFN, identify new methods to allow early assessment and considers what the treatment options are.
Definition of SFN
SFN occurs when damage to the peripheral nerves predominantly or entirely affects the small myelinated (Aδ) fibres or unmyelinated C fibres. The specific fibre types involved in this process include both small somatic and autonomic fibres. These fibres help in both thermal perception and nociception. These fibres are also involved in autonomic and enteric functions.
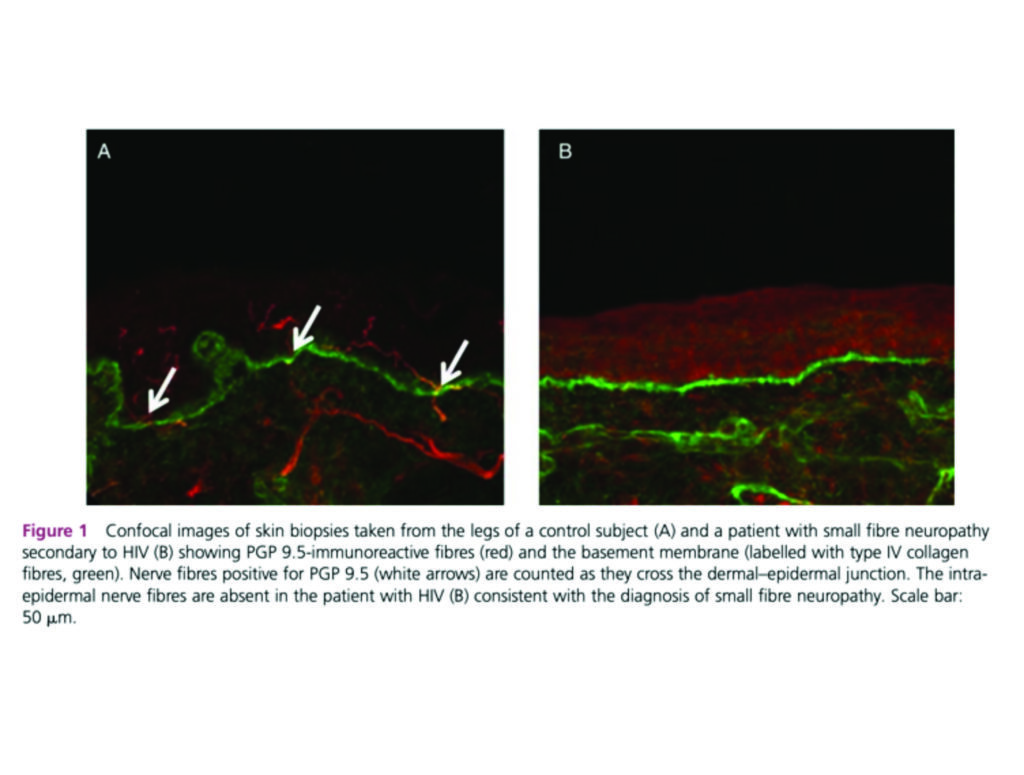
Anatomically, the small nerve fibres may be damaged or destroyed in many conditions, resulting in a loss of small nerve fibres and/or abnormal nerve fibre morphology. Figure 1 highlights skin biopsies in an individual with HIV-related SFN compared to normal skin, where the intra-epidermal nerve fibres are absent.
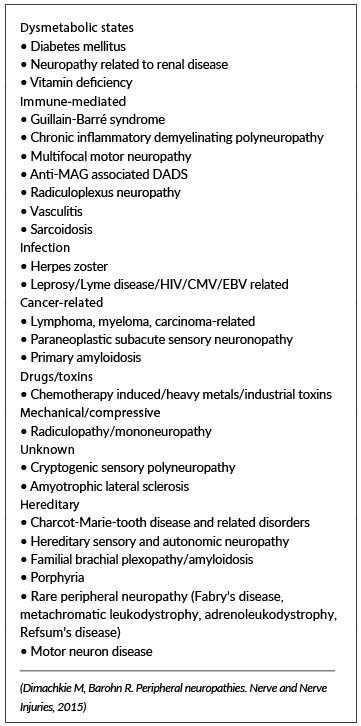
Causes of SFN
Despite extensive diagnostic evaluation, up to 50 per cent of individuals with SFN ultimately may be given a diagnosis of ‘idiopathic’. There are many other possible causes as outlined in Table 1. Attempts to subdivide causes has proven challenging and does not at present seem to offer any distinct therapeutic advantages.
Diabetes and prediabetes (including both impaired glucose tolerance and impaired fasting glucose) are frequently associated with pure SFN (15-to-23 per cent); however, concomitant large fibre involvement is seen more often. Nearly half of all subjects with idiopathic SFN have abnormal two-hour glucose tolerance tests or abnormal fasting glucose levels.
The abnormal glucose testing may be seen despite normal glycosylated haemoglobin. Several studies have also established a link between pain in SFN and abnormal glucose metabolism. There is also a large overlap between prediabetes and metabolic syndrome.
Therapy is tailored towards identification and treatment of the underlying cause of the neuropathy, when possible, while simultaneously managing symptoms of pain.
Diagnosis of SFN
a) Clinical features
The history and physical examination findings are still considered the gold standard against which all tests are compared when making a diagnosis of SFN. A detailed review of the symptoms, rate of progression, and complaints suggestive of autonomic fibre involvement are necessary.
Generally, if a patient presents with a compelling history for an SFN and an appropriate clinical exam, further testing to confirm the diagnosis may be unnecessary. This scenario is particularly likely in the context of an associated disease, such as diabetes. However, in many cases, the diagnosis may be less clear and ancillary testing may provide additional guidance.
b) Symptoms
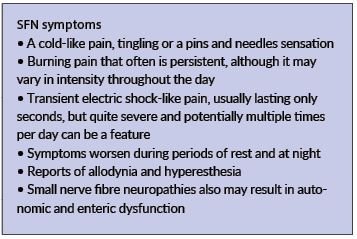
The symptoms of SFN can vary widely in severity. Many individuals report the gradual onset of distal symptoms that include vague disturbances of sensation in the affected area. Examples include:
• Symptoms such as the feeling of a wrinkle in a sock that cannot be removed or of small pebbles or sand in the shoe can be reported. Others may report a cold-like pain, tingling or a ‘pins and needles’ sensation.
• More severe symptoms of SFN may include burning pain that often is persistent, although it may vary in intensity throughout the day.
• Reports of transient electric shock–like pain, usually lasting only seconds, but quite severe and potentially multiple times per day can be a feature.
• Many symptoms worsen during periods of rest and at night.
• Many individuals report allodynia and hyperesthesia. For example, patients with SFN frequently complain that the bedsheets are exquisitely painful, and therefore wear socks or use ‘foot tents’ to keep the sheets from making physical contact with the feet.
• Small SFN also may result in autonomic and enteric dysfunction. Patients often do not identify the relationship of these symptoms to their sensory complaints; however, when asked, they may report
dry eyes, dry mouth, postural light-headedness, presyncope, syncope, abnormal sweating, erectile dysfunction, nausea, vomiting, diarrhoea, constipation, early satiety, difficulty with urinary frequency, nocturia, and/or voiding.
c) Examination
It is not uncommon to find a normal or near normal physical and neurologic examination in SFN. The coordination, motor, and reflex examinations will be normal. Light touch, vibratory sensation, and proprioception also may be normal, resulting in diagnostic confusion in some situations.
Some patients may have decreased pinprick, decreased thermal sensation, or hyperalgesia in the affected region. There may be mildly decreased vibratory sensation in some individuals. Associated skin changes in affected areas may include dry, cracked, or shiny skin, with decreased moisture on the surface of these affected areas as well. Therefore bedside assessment and diagnosis can prove quite difficult in many early cases.
d) Screen for neuropathic pain
The Neuropathic Pain Symptom Inventory differentiates various aspects of neuropathic pain, and may aid in selection of treatments. There are a number of simple neuropathic assessment tools available such as the DN4, which can help quickly identify the presence of neuropathic features.
e) Quantitative sensory testing
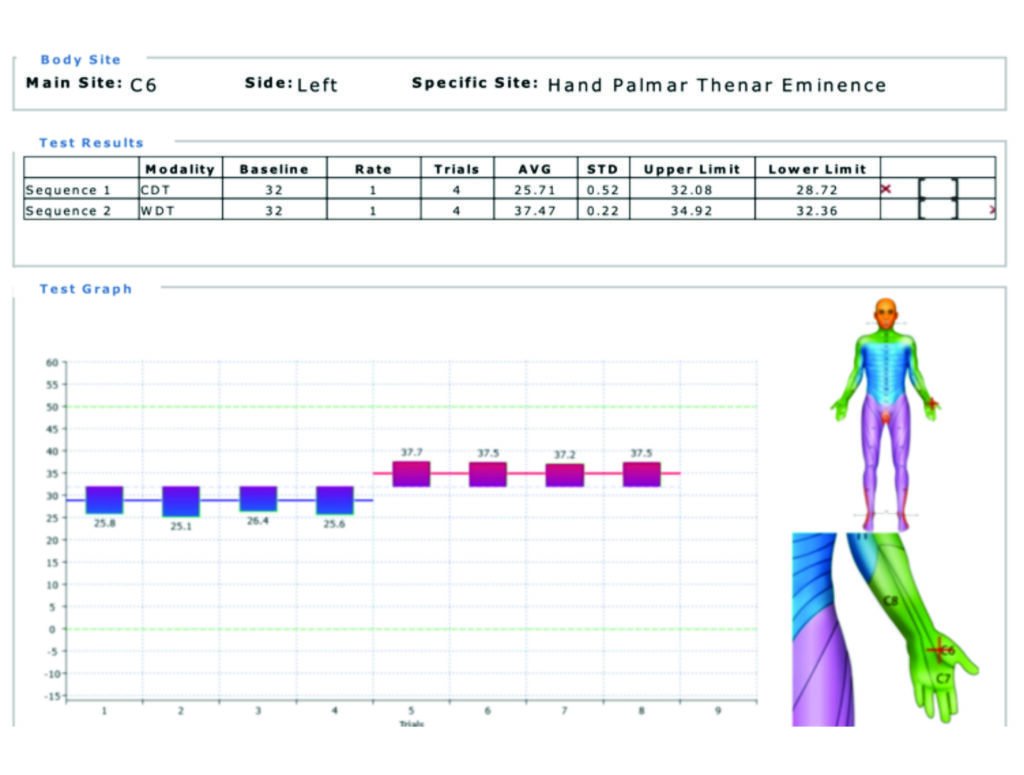
Quantitative sensory testing (QST) is an extension of the physical examination that can provide a threshold for detection of thermal sensation, thermal pain, and vibratory sensation. QST has been used in a number of longitudinal studies and clinical trials of neuropathy. In the past it was seen as a research tool but with newer technology and the ability to compare to large baseline databases, performing reliable tests in a busy clinic will become an option. Figure 2 illustrates a typical report where abnormal SFN function is reported.
There are some well-recognised limitations to QST; abnormalities in either the central or peripheral nervous system can result in the same deficit. In addition, QST requires conscious integration from the patient, and in conditions of cognitive impairment (due to disease or medication), the reliability of the test results are in question. Finally, QST is unable to distinguish between feigned and true loss of sensation.
There are few trials utilising QST in the study of isolated SFN; most trials include patients with large fibre involvement as well. Heat or heat-pain detection thresholds are considered the most useful and specific for evaluation of an SFN. Cold and cold-pain detection are transmitted through lightly myelinated Aδ fibres, while vibration detection thresholds are detected through large myelinated Aα and Aβ sensory fibres.
Recent reports of contact heat evoked potentials (CHEPs), a device that provides rapid cycles of heat resulting in evoked potentials measured by electroencephalogram, show a linear correlation between CHEP amplitude and cutaneous nociceptive nerve fibre density.
An advantage of QST testing is the non-invasive and repeatable nature of the test. Not only can it help assess the baseline status, it can be used to assess the impact treatment is having on the SFN and can guide the pharmacology management options. While further study is required to determine the utility of new variations of QST on the diagnosis of an SFN, the use of this device will influence treatment planning.
f) Quantitative sudomotor axon reflex testing
There are a variety of methods to quantify sudomotor function. The most frequently utilised and well-known test is quantitative sudomotor axon reflex testing (QSART), a measure of postganglionic sympathetic cholinergic function. This test has been used but to date no prospective controlled study has evaluated the ability of these tests to diagnose an SFN.
g) Skin biopsy
Skin biopsy is an accepted technique to investigate the structural integrity of small nerve fibres. A standard 3-mm dermatologic punch biopsy can be taken from any location on the body, but typically is performed on sites of interest in evaluation of a distal SFN (the lateral distal leg, the lateral distal thigh, and the lateral proximal thigh to look for a length-dependent pattern).
In early or mild cases of SFN, morphologic abnormalities of nerve fibres may aid in diagnosis if nerve fibre density is not reduced. Unfortunately, there are no data on the utility of skin biopsy to diagnose the aetiology of the SFN. Given the invasive nature of this test it will unlikely become standard practise.
h) Electromyography and nerve-conduction studies
Electromyography and nerve-conduction studies are well-established neurophysiologic techniques used to assess the integrity of larger myelinated sensory and motor fibres. These studies often are normal in pure SFN. If there is question of possible larger fibre involvement causing symptoms or occurring concomitantly with an SFN, these studies can clarify if larger sensory and/or motor nerve fibres are involved.
This investigation is invasive and relatively painful for the individual who may be suffering allodynia and hypersensitivity. Repeating the test to monitor progression or improvement of the condition is not offered.
i) Other investigations
Depending on each individual case it may be necessary to use advanced laboratory investigations, preform a lumbar puncture and cerebral spinal fluid analysis, or to suggest genetic testing, especially in unexplained family history.
Treatment options
Treatment of any underlying causative aetiology of an SFN is likely to be the most effective treatment of pain. However, many cases of SFN will remain idiopathic, and there is very limited evidence for specific medications in the treatment of pain from SFN.
a) Pharmacological therapy options
Most clinical studies have examined drugs in the treatment of many neuropathic pain syndromes (such as postherpetic neuralgia and painful diabetic neuropathy). In some trials, the spectrum of neuropathic pain can be broad and include diagnoses such as central neuropathic pain, radiculopathy, or carpal tunnel syndrome. These disorders may respond to treatment differently than pain from SFN.
This is a challenge when developing treatment recommendations for small nerve fibre pain because comparative effectiveness across different pain states is not known. This issue is further complicated by evidence suggesting that some diseases causing neuropathic pain respond differently to the same medications. For example, neither HIV nor chemotherapy-related neuropathic pain respond to treatments that are effective for other forms of neuropathic pain.
It is unclear if these discrepancies are methodological or due to differences in the underlying disease state. In addition, head-to-head trials of medications and long-term outcome data for SFN are lacking. When possible, disease-specific treatment guidelines should be selected for management of pain in SFN (eg, diabetes, HIV, or chemotherapy).
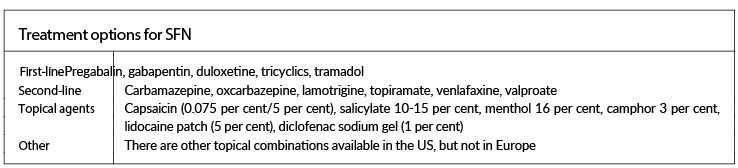
In patients with idiopathic SFN pain, physicians must select treatments based on evidence of safety, efficacy in other neuropathic conditions, tolerability, drug interactions, comorbid conditions, and cost. Table 3 outlines some options that can be considered on a case by case basis.
b) Neuromodulation management in SFN
Non-invasive and minimally invasive neuromodulation techniques may benefit some patients, but their analgesia is very limited and of short duration. Invasive neuromodulation with an implanted stimulator device may offer longer-term relief to refractory patients with poorly controlled pain. Given the prevalence of diabetic neuropathy, bioelectric modulation has the potential to be one of the commonest indications for such treatments.
While the neurophysiology of chronic stimulation and how it achieves analgesia is poorly understood the field continues to expand. Multiple spinal cord stimulation programming options are now available including tonic (t-SCS), burst (b-SCS), high frequency (HF10) and dorsal root ganglion stimulation (DRGS).
In recent months a retrospective case series examined the impact HF10 (10kHz) had on six patients with painful peripheral neuropathies, including PDPN, idiopathic polyneuropathy, and chronic inflammatory demyelinating polyneuropathy. All patients reported a reduction in verbal numerical rating scale (VNRS) pain score at last follow-up (2.7 ± 0.9) compared with baseline (7.0 ± 0.9).
Out of five patients with information available at last follow-up, two were completely off their pain medications and two reduced their dose by over 40 per cent. In summary, 10 kHz SCS treatment resulted in significant pain relief in all the patients, decreased reliance on pain medication, and improved lower limb sensory function in the majority of patients.

Conclusion
The number of individuals at risk of developing painful SFN is growing globally. Too often the symptoms are well established before any opportunity for treatment presents itself and reversing the injury is difficult with poor prognosis.
The development of practical QST assessment can provide a reliable, repeatable and non-invasive assessment tool to not only establish the presence of SFN disease, but also to track its pattern and guide pharmacology dosing regimens. Bioelectric modulation of SFN needs to be considered as an early option in the therapy regimes because it can provide long-term, drug-free solutions.
References on request
Acknowledgement
Dr Hegarty is a Consultant in Pain Management and Neuromodulation at Mater Private Hospital Cork and Clinical Director of Pain Relief Ireland. He over sees the QST assessment clinic for individuals with painful SFN and uses all modalities to offer treatment including neuromodulation for SFN.
If you feel you have individuals who could benefit from QST assessment and possible treatment options please contact info@painreliefireland.ie for an appointment.












Leave a Reply
You must be logged in to post a comment.