Atrial fibrillation (AF) is the most common cardiac arrhythmia diagnosed in clinical practice and affects
more than 33 million people worldwide. Characterised by the rapid and irregular beating of the atrial chambers of
the heart, episodes of which may become longer or continuous over time, AF is associated with a significant risk of transient ischaemic attack, ischaemic stroke, systemic embolism, and death. While not immediately life-threatening like some cardiac arrhythmias, AF carries a five-fold higher risk of stroke caused by the formation of blood clots
in the heart and blocked blood vessels in the brain.
The percentage of people with AF increases with age with 0.1 per cent under 50 years, 4 per cent between 60-to-70 years and 14 per cent over 80 years of age being affected. Due to increased lifespan, AF is on the rise and predicted to affect 14-to-17 million people in Europe by 2030, with 120,000-to215,000 new cases per year (estimated incidence 0.23-0.41 per 1,000 person/years). A further estimated 280,000-to-340,000 new ischaemic strokes, 3.5-to-four million hospitalisations for AF and 100-to-120 million outpatient visits can also be added to these figures. AF constitutes a significant public health challenge with high comorbidity and increased mortality risk and is a significant
cause of increasing healthcare costs globally. An estimated 1.4 per cent of all adults over 65 years are living with undiagnosed AF, which means more cases of unmanaged AF will create even further economic strain
on our healthcare systems.
Aetiology
AF is multi factorial in nature. Abnormalities or damage to the heart’s structure are the most common causes. AF is more common with increased age and affects certain groups of people more than others. While it can sometimes affect people who are physically very fit, AF is more common in people with other heart conditions such as hypertension, atherosclerosis, cardiomyopathy, pericarditis, heart valve and congenital heart disease. A family history of AF may also increase the risk. AF is also asso ciated with other medical conditions, such as pneumonia, lung cancer, pulmonary embolism, sarcoidosis, obstructive sleep apnoea, hyperthyroidism, and obesity. A number of triggers are associated with the condition, including smoking, excessive alcohol and coffee consumption. When no other medical conditions are associated with it, it is called lone AF.
Symptoms
AF is often asymptomatic, especially in the elderly, but many patients experience palpitations, rapid and irregular heartbeat, vague chest discomfort or symptoms of heart failure, such as weakness, light-headedness, and dyspnoea, particularly when the ventricular rate is very rapid (140 to 160 beats/ minute). Patients may also present with signs and symptoms of acute cerebrovascular accident (CVA) or other organ damage due to systemic emboli.
Diagnosis and screening
Diagnostic investigation of AF typically includes a complete medical history and physical examination, ECG, full blood count, urea and electrolytes, and serum thyroid stimulating hormone level. All patients who present with symptoms of AF should have at minimum their pulse checked for irregularities as well as a 12-lead ECG.
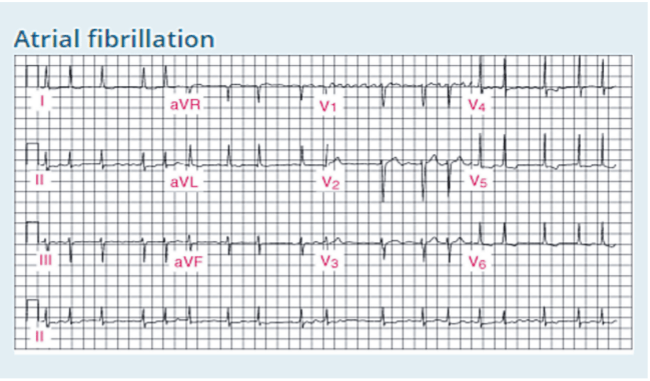
ECG findings for AF indicate the absence of P waves, irregularly irregular R-R intervals and the presence of F (fibrillatory) waves between the QRS complexes. Fibrillatory waves are irregular in timing and morphology with baseline undulations at rates up to or >300/minute, best seen in lead V1 and not always apparent in all leads. Other diagnostic tests for AF include CXR and echocardiogram. Echocardiography is carried out to assess for structural heart defects such as left atrial enlargement, left ventricular wall motion abnormalities suggesting past or present ischaemia, valvular disorders and cardiomyopathy, and to identify additional risk factors for stroke such as atrial blood stasis, thrombus or complex aortic plaque. Atrial thrombi are more likely in the atrial appendages, where they are best detected by transoesophageal rather than transthoracic echocardiography.
AF is a progressive disorder. The exactnelectro-pathological mechanisms underlying persistence of AF are at present unknown and none of the available recording techniques can determine the degree and extensiveness of atrial electro-pathology or determine the stage of the condition at any time in the process. Identifying individuals at risk of developing AF is important, however, as there is strong evidence that early detection and treatment of modifiable risk
factors can reduce morbidity and mortality.
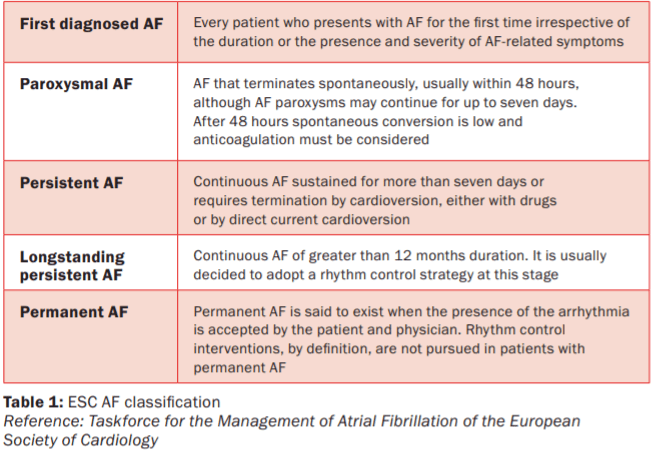
Classification of AF
The European Society of Cardiology (ESC) distinguishes five types of AF based on presentation and duration of arrhythmia (Table 1).
Complications of AF
The absence of atrial contractions in AF predisposes the patient to thrombus formation. Risk of stroke is higher in older patients and in those with mechanical heart valves, rheumatic valvular disease, hyperthyroidism, hypertension, diabetes, left ventricular systolic dysfunction, or previous thromboembolic events. Systemic emboli can also cause malfunction or necrosis of other organs. AF may impair cardiac output. Loss of atrial contraction can lower cardiac output at normal heart rate by about 10 per cent. Such a decrease is usually well tolerated except when the ventricular rate becomes too fast (>140 beats/minute), or when patients already have borderline or low cardiac output. In such cases, cardiac failure may develop.
Treatment
Treatment of AF varies from person to person and depends on the type of AF, symptoms, treatment of any underlying cause, age, and overall health. The first step is to try to find the cause of the AF. For example, hyperthyroidism can sometimes be the underlying cause of AF and medication to control the condition can correct the symptoms of AF.
If no underlying cause is found, treatment of AF is usually aimed at either rhythm or rate control. Since AF induces electrical, structural, and contractile remodelling, therapy aimed at prevention or restoration of remodelling and consequently restoration of sinus rhythm should be the first choice strategy. The different AF treatment modalities include pharmacological therapy, electrical cardioversion, pacemaker implantation combined with His bundle ablation or surgical isolation of the pulmonary veins with or without additional linear lesions/substrate modification. Anti-arrhythmic medication can control AF by restoring normal heart rhythm and controlling the rate at which the heart beats. The choice of anti-arrhythmic depends on the type of AF, comorbidities, side-effects of the medicine chosen and response to therapy. Some patients may need more than one anti-arrhythmic to control AF and a variety of medications are available to restore normal heart rhythm.
The aim is to reduce the resting heart rate to <90 beats per minute, however, in some people the target is <110bpm. First-line medications include beta blockers, such as propranolol or atenolol and nondihydropyridine calcium channel blockers such as diltiazem or verapamil, which slow the conduction of impulses to the ventricles. Digoxin may be added to control the heart rate further. Digoxin is usually only effective for controlling the ventricular rate at rest and should therefore only be used as a monotherapy in predominantly sedentary patients.
Anti-arrhythmic drugs can be classified clinically into those that act on supraventricular arrhythmias (eg, verapamil), both supraventricular and ventricular arrhythmias (eg, amiodarone), and those that act on ventricular arrhythmias (eg, lidocaine).
They can also be classified according to their effect on the electrical behaviour of myocardial cells during activity (Vaughan Williams Classification). Class I anti-arrhythmic medications are membrane stabilising drugs, eg, lidocaine and flecainide. Class II are beta blockers. Class III include amiodarone and sotalol and Class IV are calcium channel blockers, including verapamil, but not dihydropyridines. The negative inotropic effects of anti-arrhythmic drugs tend to be additive. Special care should be taken if two or more are used, especially if myocardial function is impaired. Many medications that are effective in countering arrhythmias can also provoke them in some instances.
Patients must be monitored closely as common side-effects of anti-arrhythmic medications include hypotension, fatigue, nausea, vomiting, possible issues with liver, kidneys, thyroid or lungs, and heart rhythm disorders. Amiodarone causes sensitivity to sunlight and skin changes are common, therefore, sun protection is important when taking this medication. Aspirin is not recommended to prevent strokes caused by AF. Patients with a high or moderate level of risk of stroke or thrombus formation due to AF are usually prescribed an anticoagulant, such as the vitamin K antagonist warfarin or a novel oral anticoagulant (NOAC), also called direct oral anticoagulants (DOACs), such as dabigatran, rivaroxaban, apixaban or edoxaban. There are fewer dietary and medication interactions with the newer agents and less need for monitoring. NOACs are at least as effective or superior to warfarin for stroke prevention in patients with non-valvular AF, and are at least as safe or safer in terms of bleeding risk, according to three large clinical trials. NOACs have major pharmacologic advantages over warfarin, including rapid onset/offset of action, few drug interactions and predictable pharmacokinetics.
Practical advantages of NOACs over warfarin include fixed once- or twice-daily oral dosing without the need for coagulation monitoring.Potential drawbacks include a risk of bleeding that might be increased in patients over 75 years of age, the lack of a routine laboratory test to reliably measure anticoagulant effect and previously the lack of an antidote for reversal. Choice of NOAC is influenced by the patient’s individual characteristics, including risk of stroke or VTE, risk of bleeding, and comorbidity, in particular renal dysfunction. It is also recommended that one of the bleeding risk scores, such as the HAS-BLED score, be used to help risk-assess patients. The choice of agent should take into consideration the patient’s age, weight and creatinine clearance, as dose adjustment may be required. Cardioversion is a medical procedure carried out in a hospital setting by which a tachycardia or other cardiac arrhythmia is converted to a normal rhythm using an electric current or pharmacology. Synchronised electrical cardioversion uses a therapeutic dose of electric current to the heart at a specific moment in the cardiac cycle, restoring the activity of the electrical conduction system of the heart. Electrical cardioversion has success rates of between 65-to-90 per cent and can be performed safely as a day case procedure. The likelihood of success can be further improved by the co-administration of anti-arrhythmic drug therapy.Pharmacologic cardioversion, also referred to as chemical cardioversion, uses anti-arrhythmic medication instead of an electrical shock. Pharmacological cardioversion is often used for the treatment of AF of recent onset. Flecainide, ibutilide, propafenone, and vernakalant can be used in patients with no structural heart disease. Where structural heart disease is present, intravenous amiodarone is the drug of choice. Flecainide administered intravenously in patients with AF of recent onset has been shown to restore sinus rhythm in 72-to-95 per cent of patients, with greatest success rates in those who receive treatment within 24 hours of AF onset.
When AF has persisted for >48 hours, pharmacological cardioversion is much less likely to be effective. Amiodarone appears to be the most effective agent for restoring sinus rhythm in patients with persistent AF. For patients who have been in AF for longer than 12-to-24 hours, or in whom the duration of the arrhythmia is not clear, a minimum period of anticoagulation of three weeks is recommended before cardioversion. Even if echocardiography has demonstrated no thrombus before cardioversion, patients should be anticoagulated for at least one month post cardioversion, since mechanical atrial function may return slowly after cardioversion.
Risk of stroke is higher in older patients
and in those with mechanical heart
valves, rheumatic valvular disease,
hyperthyroidism, hypertension, diabetes,
left ventricular systolic dysfunction,
or previous thromboembolic events
Catheter ablation has emerged as an important rhythm-control strategy and is an increasingly used option if medical treatment for AF has not been successful. The procedure restores the heart’s normal rhythm by electrically isolating the left atrium from the pulmonary veins where most abnormal electrical activity that promotes AF originates. The procedure destroys or ablates the diseased area of the heart and interrupts abnormal electrical circuits. The principal reason to place a pacemaker in a patient with AF is to treat symptomatic bradycardia, especially due to sick sinus syndrome (tachycardia-bradycardia syndrome). This tends to be mainly used in people aged 80 or over
Differentiation between atrial flutter and AF Atrial flutter is much less common than AF, but its causes and haemodynamic consequences are similar. About a third of people with atrial flutter also have AF. Both conditions carry increased risk of stroke. In AF, the atria beat irregularly. In atrial flutter, the atria beat regularly, but faster than usual and more often than the ventricles, and there may be four atrial beats to every one ventricular beat. During atrial flutter, unlike AF, electrical activity in the atria is coordinated. The atria do contract, but at a very rapid rate of 250 to 350 times per minute.
This rate is too fast to allow every impulse to be conducted through the atrioventricular node to the ventricles. Because the AV node cannot usually conduct at this rate, typically half of the impulses get through (2:1 block), resulting in a regular ventricular rate of 150 beats/minute. The diagnosis of atrial flutter is by electrocardiography. In typical flutter,
ECG shows continuous and regular atrial activation with a saw tooth pattern, most obvious in leads II, III, and aVF.
Carotid sinus massage can increase AV block and better expose the typical flutter waves. A similar response may follow pharmacologic AV nodal blockade, example with adenosine, but such therapy does not terminate atrial flutter.
Treatment of atrial flutter focuses on ventricular rate control, rhythm control, and prevention of thromboembolism. Patients with chronic or recurrent atrial flutter require an oral anticoagulant. The choice among the therapies
is based on the same considerations as for AF. Patients often require cardioversion or atrial flutter substrate ablation.
AF is associated with one-in-three strokes in Ireland AF is the most common cardiac arrhythmia affecting at least 3 per cent of Irish adults over 60 and is associated with almost onein-three strokes in Ireland. Globally the Risk of stroke is higher in older patients and in those with mechanical heart valves, rheumatic valvular disease, hyperthyroidism, hypertension, diabetes, left ventricular systolic dysfunction, or previous thromboembolic events prevalence of AF is rising and approximately 600 men and 375 women per 100,000 population are affected. In the 2017 report Burden of Stroke in Europe, the Stroke Alliance for Europe signalled a possible 58 per cent increase in the absolute number of strokes in Ireland over the next decade. Much of this can be accounted for by increasing numbers of AF-related stroke as our population ages.
The population aged 65 years and over in Ireland is increasing at the rate of 4 per cent annually. The CSO predicts that people aged over 65 will represent between 21.5-to-27.9 per cent of the Irish population by 2046. As a result AF is a growing public health concern. Screening for AF in general practice Projections show that the number of people living with stroke as a chronic condition will rise from 3,718,785 in 2015 to 4,631,050 in 2035, representing an increase
of 25 per cent or almost one million people across Europe. There are approximately 8,000 strokes in Ireland annually, a third of which are associated with AF.
In 2015, HIQA published a health technology assessment (HTA) of a national screening programme for AF in primary care. The HTA announced that a national screening programme for AF for over-65s in primary care would be cost-effective. HIQA’s Director of Health Technology Assessment Dr Máirín Ryan, said: “Based on the best available evidence, annual opportunistic pulse palpation for those aged 65 and older is expected to lead to reductions in the incidence and severity of AF-related strokes assuming that those detected by screening have a comparable risk of stroke as those detected through routine care.”
Analysis estimated that screening would result in the detection of one additional AF case for every 22 people screened from age 65 onwards, and one stroke avoided for every 270 people screened over the same period. The total incremental cost to the HSE was estimated at €3.7 million over the first five years. This included the additional costs associated with screening, ECGs and AF drug therapy in diagnosed cases, as well as the cost savings resulting from a
gradual decrease in stroke incidence over a period of five years.
Almost 11 per cent of Irish adults aged 65 years and over attending general practice have AF. The HSE (2015) study on atrial fibrillation screening in general practice also concluded that “opportunistic screening for an irregular pulse in general practice to assist in the detection of AF is both feasible and beneficial”. This multi-site prospective
observational study found that approximately 8,415 new cases of AF could be identified by opportunistic screening in general practice each year.
Each GP could expect to diagnose 17 new cases per 1,000 patients annually through opportunistic screening in
practice. General practice is well placed to opportunistically screen older patients and is ideal in terms of the pathway for treatment for those identified. Opportunistic screening for an irregular pulse carried out by GPs
and general practice nurses (GPNs) “has the potential to be an extremely important stroke prevention strategy, capable of saving society and the health service significant social and economic costs”.
References on request












Leave a Reply
You must be logged in to post a comment.