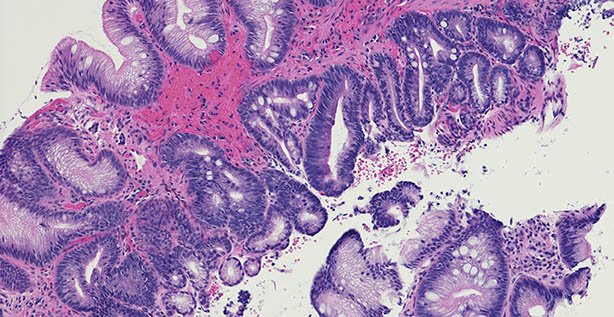
Microscopic photo of a professionally prepared slide demonstrating intestinal metaplasia of the esophagus. Barrett's esophagus caused by gastroesophageal reflux disease. Chronic esophagitis.
High-dose proton pump inhibitors (PPI)
and aspirin chemoprevention therapy, especially in combination, significantly
and safely improved outcomes in patients with Barrett’s oesophagus, according
to the findings of a major trial in the area, the results of which were relayed
to the ISG Winter Meeting by one of the study’s key authors.
Oesophageal adenocarcinoma is the sixth-most common cause
of cancer death worldwide (five-year survival is less than 10 per cent), and is
on the rise, with Barrett’s oesophagus the biggest risk factor.
As
aspirin reduces inflammation and PPIs reduce acid reflux, the Aspirin and
Esomeprazole Chemoprevention in Barrett’s metaplasia Trial (AspECT) aimed to
evaluate the efficacy of high-dose esomeprazole PPI and aspirin for improving
outcomes in patients with Barrett’s oesophagus, explained lead author Prof
Janusz Jankowski, Senior Consultant Physician, University Hospitals Morecambe
Bay, UK.
AspECT is
the first randomised trial to evaluate PPI and aspirin chemoprevention in
Barrett’s oesophagus and the largest randomised trial of Barrett’s oesophagus
ever done, with 20,095 participant-years of follow-up in 2,557 patients. The
trial was carried out at 84 centres in the UK and one in Canada. Patients with
Barrett’s oesophagus of 1cm or more were randomised 1:1:1:1 to receive
high-dose (40mg twice-daily) or low-dose (20mg once-daily) PPI, with or without
aspirin (300mg per day in the UK, 325mg per day in Canada) for at least eight
years, in an unblinded manner.
The
primary composite endpoint was time to all-cause mortality, oesophageal
adenocarcinoma, or high-grade dysplasia.
Between
2005 and 2009, patients were recruited and median follow-up and treatment
duration was 8.9 years.
High-dose
PPI (139 events in 1,270 patients) was superior to low-dose PPI (174 events in
1,265 patients).
Combining
high-dose PPI with aspirin had the strongest effect compared with low-dose PPI
without aspirin (TR 1.59, 1.14–2.23, p=0.0068). The numbers needed to treat
were 34 for PPI and 43 for aspirin. Only 28 (1 per cent) participants reported
study-treatment-related serious adverse events.
Further
work is needed and the AspECT Excel Trial aims to provide longer-term answers.
Speaking
to the Medical
Independent,
Prof Jankowski said the idea of a ‘magic bullet’ is never going to happen, and
it is about balancing benefits and risks in an appropriate way.
“Do I
think you should be on life-long PPIs without anyone ever reviewing you?
Categorically not; that would be insane. What we are saying is that for people
with significant symptoms of reflux disease, not only do they show efficacy,
but we think that long-term PPI use, at least until nine years, is relatively
safe. There may be a low incidence of side-effects but we haven’t seen that,
though it could change with another five-to-10 years of follow-up.
“Secondly,
the combination of low-dose aspirin with PPIs is remarkably safe and there is
evidence of increased efficacy there” [in terms of preventing side-effects from
sudden death and aspiration and bronchopneumonia].
“The third aspect, and the jury is
still out, is whether low-dose aspirin can actually prevent cancer. We didn’t
actually show any cancers being prevented, but we’ve seen the ‘smoking gun’ in
the sense that low-dose aspirin particularly seemed to have an effect on the
premalignant lesions of high-grade dysplasia and we are reasonably confident
that if there had been follow-up for another five or 10 years, we’d be able to
see that breakthrough in preventing oesophageal adenocarcinoma.”





Leave a Reply
You must be logged in to post a comment.